Benzyne, Arynes & Nucleophilic Aromatic Substitution
Introduction
In the previous blog post, I looked at two different methods to achieve nucleophilic substitution on benzene derivatives. The first method is the classic nucleophilic aromatic substitution involving an addition-elimination mechanism. It required the addition of a nucleophile to an activated aromatic ring prior to the leaving group being kicked out. The second substitution was closer to an SN1 reaction or elimination-addition. In this reaction, a diazonium salt breaks down to give an aryl cation or aryl radical before the addition of the nucleophile. These two possibilities are summarized below:
Two possible reactions that bring about nucleophilic aromatic substitution. The top reaction is classical nucleophilic aromatic substitution involving an activated benzene ring that has a leaving group either ortho or para to an electron withdrawing group. The reaction proceeds by an addition-elimination mechanism and the Meisenheimer complex. The second reaction is closer to SN1 substitution. It starts from a diazonium salt that eliminates to give an aryl cation. This is trapped by the nucleophile in an elimination-addition mechanism.
There is a third method that substitutes a leaving group on a benzene derivative. This is a brutal reaction involving highly basic nucleophiles in an elimination-addition mechanism.
The synthesis of phenol from chlorobenzene. Nucleophilic substitution occurs through an elimination-addition mechanism.
This third form of nucleophilic aromatic substitution occurs through an intermediate called benzyne. It is an odd looking species that participates in an number of intriguing reactions. In this post, I will focus solely on substitution and give the bare essentials required at undergraduate level. Those that want to know more about the chemistry of arynes could do a lot worse than starting with these reviews HERE, HERE & HERE.
The Mechanism
In the post on nucleophilic aromatic substitution, I said it was impossible to directly substitute a halide on a benzene ring, and this is still true whatever the scheme above looks like! So, how can the hydroxide add to benzene if you have not activated the ring by the addition of electron withdrawing groups or used a diazonium salt that spontaneously eliminates nitrogen gas prior to the nucleophilic attack? A clue to what is happening comes from the following reaction. This uses a strong base/nucleophile and gives two regioisomers in, more or less, equal amounts.
The reaction of p-bromotoluene with sodamide to give 4-methylaniline (para-toluidine) and 3-methylaniline (meta-toluidine).
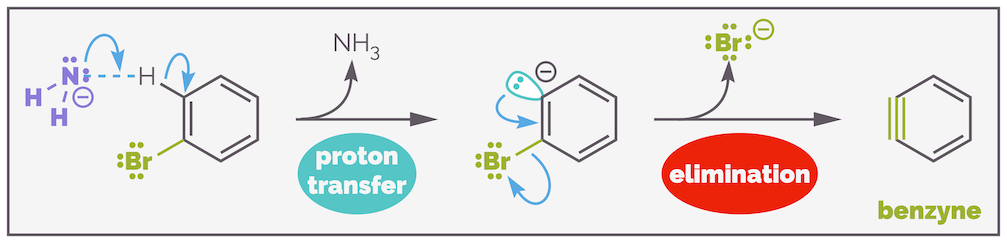
The formation of the two products suggests that there is a common intermediate that permits the nucleophile to add at two different carbon atoms. This intermediate is called benzyne and is formed by the deprotonation of the aromatic ring and elimination of the halide leaving group. This is step 1 or the elimination step.
The formation of benzyne by deprotonation ortho to a leaving group and then elimination (step 1).
Step 1 - elimination
Deprotonation of the benzene ring is hard and this is the slow, rate determining step of this substitution reaction. Only the protons α to the leaving group can be removed. The leaving group, a bromine atom in this example, is electronegative, and this permits inductive stabilization to the resulting negative charge. This is a weak effect as the bromine is not a strong electron withdrawing group. The deprotonation leaves a lone pair of electrons in an sp2 orbital, which is is perpendicular to the π bonds so is incapable of delocalization. But it is in the same plane as the C–Br bond allowing minimal stabilization by the electronegative bromine (I've just repeated myself!).
The elimination of the leaving group to form a new π bond is another challenging step as the anion and the leaving group are syn-periplanar. This doesn't permit as good orbital overlap as found in the anti-periplanar arrangement of E2 elimination. But elimination can occur and the resulting molecule is benzyne. It is odd as it is effectively putting an alkyne in a six-membered ring. The standard bond angles of an sp hybridized atom (180°) just don't like this!
The new π bond is very different to a standard π bond. Up until this point, every π bond you will have seen will have been made from the side-to-side overlap of two parallel 2p orbitals. Such an overlap is relatively good (not as good as the head-to-head overlap of orbitals to form σ bonds but that is why π bonds are functional groups). Arguably, there is one such π bond in the ring of benzyne as shown below (oranage and blue orbitals). This π bond is parallel to the other p orbitals and is part of the aromatic system. The new π bond (green orbitals) is perpendicular to the aromatic ring, it sticks outside the ring or is external to the ring. It is made by the poor overlap of two sp2 orbitals. These orbitals are not parallel, there is poor overlap and the bond is weak or highly reactive.
The structure of benzyne. Benzyne has a triple bond or alkyne in the ring. This is made up of a σ bond, and two π bonds, one of which is 'normal' being formed from the overlap of 2p orbitals. The other is 'abnormal', it is made from the weak or poor overlap of two sp2 orbitals that are splayed away from each other. This puts the new π bond outside the ring and perpendicular to the π system that gives the ring its aromaticity.
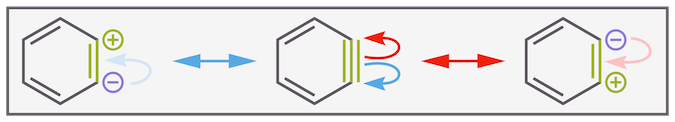
Sometimes it is useful to think of the resonance structures of benzyne. Two of these have charges on adjacent carbon atoms. To a certain extent, they explain why benzyne acts as an electrophile. The carbocation character instilled by the resonance structures allows nucleophilic attack. But, please remember that these are just resonance structures and not real. The structure of benzyne is best thought of as a triple bond, just a very weak and reactive triple bond.
Potential resonance structures for benzyne. These reveal that the triple bond is more electrophilic than normal alkynes. While the resonance structures are useful, the structure of benzyne is closer to a triple bond, just a very weak triple bond!
Step 2 - addition
The second step of the reaction is the addition of the nucleophile. The resonance structures above show that the triple bond is electrophilic. Benzyne is highly reactive and this process is fast. It results in the formation of an anion that is protonated to give the final product.
The second step of the process is the addition of the nucleophile to the benzyne. This gives an aryl anion that is protonated to give the product.
Under such strongly basic conditions there is an additional step that is invariably ignored in textbooks, and that is the base will deprotonate the amine (aniline). The resulting anion is stabilized over the aromatic ring. At the end of the reaction, you need to neutralize the solution if you want to isolate the product (the fact these two steps cancel each other out probably explains why it is ignored but if you are actually doing the experiment in a lab it is vital).
The formation of benzyne requires strongly basic conditions. This means any acidic protons will undergo deprotonation. At the end of the reaction it is necessary to neutralize the reaction mixture with acid.
The formation of the triple bond explains the mixture of products observed at the start of this section. If you invoke the resonance structures from earlier, there are two possible cations and the the nucleophile can add to either (it can add to either end of the triple bond of the aryne) to give the two different regioisomers.
The formation of the two isomers of toluidine (methylaniline) can be explained by the aryne intermediate. The nucleophile can attack either end of triple bond to give the two different isomers.
Overall mechanism
The benzyne mechanism for nucleophilic aromatic substitution is given below. It is an elimination-addition process. Depending on the nature of the nucleophile/base there might be additional proton transfer steps at the end.
The complete mechanism for nucleophilic aromatic substitution proceeding through a benzyne/aryne intermediate.
Influence of Substituents
Most of the previous discussion has centered on the chemistry of benzyne, this is the reactive intermediate made from benzene with a single leaving group attached. As soon as there is a substituent on the ring the molecule is no longer benzyne (although lots of us use this term as shorthand), and the resulting triple bond species is an aryne (ar- from arene & -yne from alkyne or triple bond) but you tend to see the terms used interchangeably.
Benzyne is derived from benzene. Arynes are all the other aromatic derivatives.
The nature of any substituent on the aromatic ring can influence both the formation of the aryne and which carbon atom is attacked by the nucleophile. In other words, the R group (whether it is electron donating or electron withdrawing, bulky or small) can control regiochemistry and determine which isomer is formed. The explanation below is the simplified version of predicting regioselectivity. It is the reasoning found in the majority of undergraduate textbooks (that's if they even bother to mention this!). It is becoming clear that, as always, the selectivity may have more subtle origins, and the aryne distortion model has been proposed by Garg and Houk. If you are interested in more details start HERE.
This is one of the few times that the origin of the selectivity has nothing to do with delocalization or resonance. The sp2 orbitals that overlap to make the reactive triple bond are perpendicular to the aromatic ring. This means there is no overlap with the 2p orbitals that form the π system. If there is no overlap of orbitals, there can be no delocalization or sharing of electrons. This means the substituent, the R group, can only interact with the triple bond through inductive effects (σ delocalization or the electronegativity of the various substituents) or through steric effects, it can slow the approach of the nucleophile.
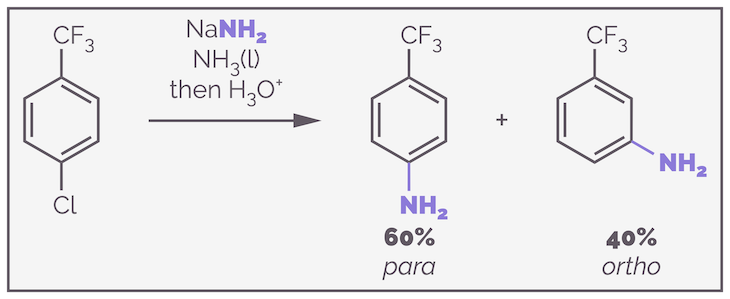
An example that demonstrates the inductive effect in action is shown below with the substitution of a trifluoromethyl derivative.
The strong electron-withdrawing effect of the trifluoromethyl substituent slightly encourages the formation of the para substituted isomer.
The reaction proceeds by the standard mechanism and there is only one possible aryne that can be formed (the molecule is symmetrical so deprotonation of the proton ortho to chlorine atom on either the left or the right gives the same aryne - don't get fooled by the placement of the double bonds in the drawing above, this is irrelevant, the π electrons are delocalized). Addition to the aryne can give two different anions. The anion that has the lone pair of electrons closer to the electron withdrawing trifluoromethyl group is favored. The negative charge is more stable as it is nearer the most electronegative elements.
The slightly selectivity in this substitution reaction can be understood by considering the stability of the anionic intermediate formed on addition of the nucleophile. If the nucleophile adds to the meta position the anion is as far from the stabilizing effect of the trifluoromethyl group as it is possible to get. If the addition occurs at the para position, the anion will be meta and this is closer to the inductive effect caused by the fluorines. It is more stable and is favored.
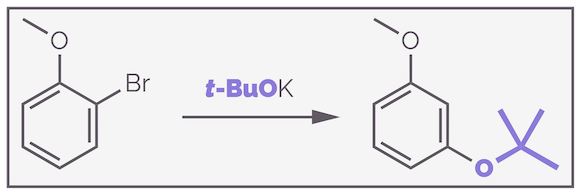
Steric effects can also influence the selectivity. The following substitution gives exclusively the meta isomer.
Substitution of ortho-bromoanisole with tert-butoxide gives predominantly the meta product.
The selectivity can be rationalized both by the inductive effect and the influence of steric hindrance. Elimination can give only a single aryne as there is only one proton ortho to the bromide. The alkoxide nucleophile could attack either ortho to the methylether or meta to it. The latter leads to the anion being closer to the electron withdrawing oxygen atom (ortho to it) and this is favored.
The meta selectivity can be justified by the inductive argument. The nucleophile adds to form the anion closest to the electronegative oxygen atom as this is the more stable anion.
In this case, there is also a steric factor at work and this might explain why virtually none of the ortho product is observed. Simply put, the methyl ether blocks the approach of the nucleophile discouraging attack at the ortho position.
Steric hindrance can also influence the regioselectivity of nucleophilic attack. The nucleophile avoids interaction with the ether if it attacks at the meta position.
Conclusion
The benzyne/aryne mechanism is the third and final version of nucleophilic aromatic substitution that I will discuss. This post has just been a brief introduction to this interesting chemistry. If your nucleophile is also a strong base it is possible that it can deprotonate the ortho hydrogen next to a leaving group. If this occurs elimination to give a triple bond on the outside of the aromatic ring is possible. The triple bond is very weak or highly reactive as a result of the poor overlap of the two sp2 hybridized orbitals. The triple bond is electrophilic, and the nucleophile can add to give a product that looks like a substitution has occurred. In reality, the reaction was elimination and then addition. The nucleophile can add to either end of the triple bond and this can lead to regioisomers. Any substituent on the aryne can influence the regiochemistry. Simplistically, this is achieved by a combination of inductive and/or steric effects. More accurately, this might be due to a model known as the aryne distortion model.
It should come as no surprise that the benzyne or aryne triple bond can participate if other reactions and not just nucleophilic addition. It is a reactive species in cycloadditions, and allows the rapid construction of complex molecules. If you are interested in this chemistry I recommend these reviews HERE, HERE & HERE.