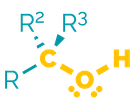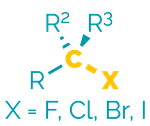Functional Groups
Introduction
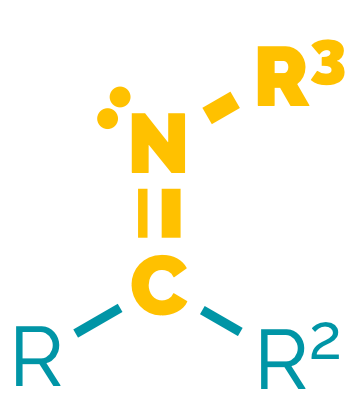
A functional group is a collection of atoms that imparts characteristic properties to a molecule. These properties are often surprisingly independent of the rest of the molecule. Normally, a functional group is defined as the atoms attached to a single carbon atom, although it can be larger. The majority of functional groups involve a carbon with an electronegative atom attached. Exceptions are alkenes and alkynes along with organometallics.
The functional group approach to organic chemistry is based around learning the reactivity of a few functional groups and using these to predict the chemistry of thousands of molecules. We prefer a slightly different approach based around electrons and the polarisation of bonds.
Sorry, I had an issue with the pictures - I’ll correct this at some point soon(ish)
Alkanes
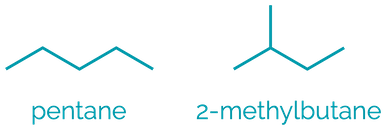
Name = -ane
Example = butane
Examples:
Alkanes are the simplest hydrocarbon comprising of just C–C and C–H single bonds. They are non-polar, hydrophobic compounds with limited reactivity.
Alkenes
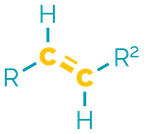
Name = -ene
Example = 2-butene or but-2-ene
Examples:
Alkenes are the ‘first’ functional group and contain a double (π) bond between two carbon atoms. They are non-polar and hydrophobic but are more reactive than alkanes. They are good nucleophiles in addition reactions and their chemistry can be modified by other functional groups.
Alkyne
Name = -yne
Example = 2-butyne or but-2-yne
Examples:
Alkynes are non-polar and hydrobobic. They participate in the same reactions as alkenes but are more reactive. This means they are good nucleophiles in addition reactions (π nucleophile). Terminal alkynes, those with a C–H bond, can be deprotonated to make an anion or ‘σ nucleophile’.
Arene (benzene derivatives)
Name = -benzene (amongst others)
Example = methylbenzene
Examples:
A large family of molecules and it is hard to generalise. Substituted benzenes can be polar and hydrophilic. They can have strong non-covalent interactions with each other. These are known as π-π-interactions (dispersion forces). The most common form of reactivity is electrophilic aromatic substitution.
Amines
Names = amino- or -amine
Examples = 2-aminoethanol or ethanamine
Examples:
Amines are polar compounds that frequently participate in hydrogen bonding both as H-bond acceptor (lone pair) and H-bond donor (N–H). They are hydrophilic and often have an unpleasant ‘fishy’ smell. They are Brønsted bases, with the lone pair accepting a proton. They are good nucleophiles.
Alcohols
Name = hydroxy- or -ol
Examples = 2-hydroxyethanal or ethanol
Examples:
Alcohols are polar molecules that readily form hydrogen bonds both as acceptor and donor. They are often hydrophilic with high boiling points. They are less basic than amines due to increased electronegativity. This means they are poorer nucleophiles. They will participate in substitution and elimination reactions.
Halides
Name = halo-
Example = bromobutane
Examples:
Halides are polar, hydrophobic compounds that frequently have high melting or boiling points (reflecting the size of the halogen atoms). They are good electrophiles and are often substrates in substitution and elimination reactions.
Thiols
Name = mercapto- or -thiol
Example = mercaptobenzoic acid or cyclopentanethiol
Examples:
Thiols are best known for their smell. Many smell vile (skunk spray) but a number are very pleasant (the smell of roast coffee). Thiols are the sulfur equivalent of an alcohol but they are poor hydrogen bond donors and acceptors. This means they are often volatile and less water soluble. They are good nucleophiles and readily form sulfur-centred radicals, which is important for the synthesis of disulfide bridges.
Aldehydes
Name = oxo- or -al
Example = 3-oxopropanoic acid or methanol
Examples:
Aldehydes are polar molecules that are often good hydrogen bond acceptors. Their chemistry is characterised by nucleophilic addition to the carbon of the carbonyl group. They can be protonated.
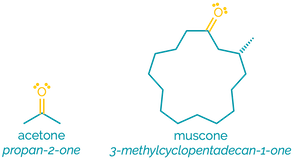
Ketones
Name = oxo- or -one
Example - 2-oxopropanal or propanone
Examples:
Ketones are similar to aldehydes although they are slightly less polar and less reactive. Like aldehydes they are hydrogen bond acceptors and can be protonated. Although less reactive than aldehydes, they are still good electrophiles and participate in addition reactions.
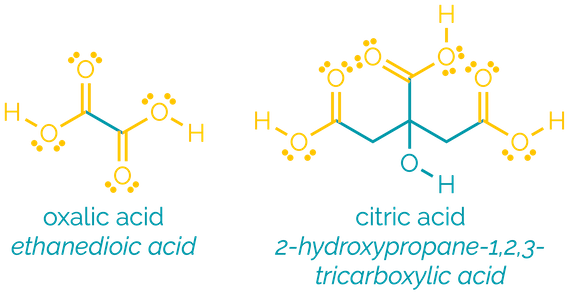
Carboxylic acids
Name = -oic acid or -carboxylic acid
Example = ethanoic acid or cyclohexanecarboxylic acid
Examples:
Carboxylic acids are polar and readily participate in hydrogen bonding. They are frequently found as dimers, hydrogen bonding to another molecule, and this gives them high boiling points. Smaller carboxylic acids are water soluble. They are Brønsted acids, donating a proton. After proton transfers, the most common reactions of the acids are acyl substitution reactions.
Amides
Name = [if ≠ H then (N-alkyl)-] & -amide
Example = butanamide or N-methylbutanamide
Examples:
Amides are a carboxylic acid derivative. When part of a protein they are known as peptide bonds. Amides are polar. They are hydrogen bond acceptors through the oxygen atom and can be hydrogen bond donors if they have an N–H bond. They are less hydrophilic than carboxylic acids. They are neither particularly acidic or basic. They are the least reactive of the common carboxylic acid derivatives (ignoring carboxylate anions) due to delocalisation of the nitrogen lone pair but they will still participate in acyl substitution reactions.
Esters
Name = alkoxy- -oate (carboxylate)
Example = ethyl ethanoate or ethyl cyclohexanecarboxylate
Examples:
Esters are another carboxylic acid derivative. They are polar but less polar than acids and alcohols. They are hydrogen bond acceptors but not donors. They are more volatile than acids but less water soluble. Their reactivity is dominated by acyl substitution reactions but they are also electrophiles in addition reactions.
Less common functional groups (at undergraduate level)
Nitro group
Name = nitro-
Example - nitromethane
Examples:
The nitro group is highly polar. It is a strong electron withdrawing group. This makes the α-protons acidic so they can be deprotonated to create a good nucleophile (the Henry reaction). It is relatively easy to introduce one nitro group onto an aromatic ring. The nitro group is readily reduced to an amine. Nitro groups are the most common explosophore.
Ethers
Name = alkoxy- or alkyl alkyl ether
Example = methoxyethane or methyl ethyl ether
Examples:
Ethers are relatively non-polar and have low boiling points. They are hydrogen bond acceptors and smaller ethers are slightly water soluble. They are fairly inert, used as solvents in reactions rather than reagents. Epoxides (oxiranes) are three membered rings comprising one oxygen and two carbon atoms. These special ethers are more reactive to allow the release of ring strain.
Sulfides (thioethers)
Name = alkylsulfide
Example = dimethylsulfide
Examples:
Sulfides or thioethers are the sulfur equivalent of an ether. They are less polar but more polarisable. They are less volatile and less hydrophilic. Small sulfides tend to have bad smells. They are readily oxidised but their reactivity is rarely discussed at undergraduate level.
Disulfides
Name = alkyl alkyl disulfide
Example = ethyl methyl disulfide
Examples:
Disulfides are not that common in organic chemistry. They are even less common in undergraduate courses. They are very important in the structure of proteins. Disulfide bridges between two peptide chains or within a single chain are common motifs in tertiary protein structure.
Acetal (ketal)
Name = dialkoxy- or parent-carbonyl suffix dialkyl acetal
Example = 1,1-dimethoxypropane or propanal dimethyl acetal
Examples:
An acetal is a functional group that includes two ethers attached to the same carbon. They are derived from aldehydes or ketones. Acetals formed from ketones are sometimes called ketals, indicating that there is no hydrogen on acetal carbon. Most glycosidic bonds in carbohydrates and polysaccharides are acetals. The key reaction of acetals is hydrolysis. Acetals are relatively stable requiring acid-mediated hydrolysis to break them down to alcohol and aldehyde (or ketone).
Imine
Name = -imine
Example = N-methylethanimine
Examples:
Imines are the nitrogen equivalent of an aldehyde or a ketone. They are sometimes called Schiff bases. Imines are less polar than the analogous carbonyl compounds. They are weakly basic and can be protonated on the nitrogen to give an iminium ion. Their reactions are dominated by nucleophilic addition to the C=N bond, and participate in similar reactions to aldehydes/ketones. They readily undergo hydrolysis, the addition of water to give the aldehyde (or ketone) and an amine.
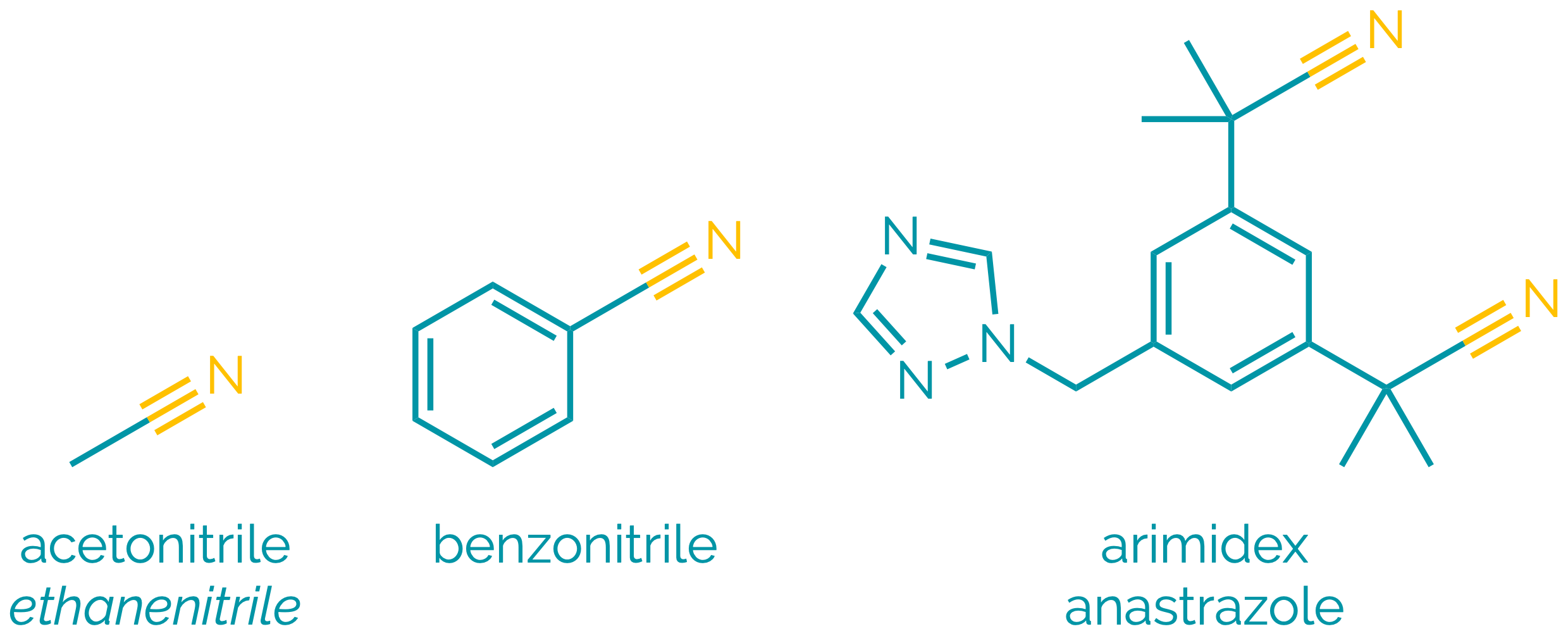
Nitriles
Name = cyano- or -nitrile
Example = 3-cyanopropanoic acid or ethanenitrile or benzonitrile
Examples:
Nitriles are polar compounds. The polarisation of the C≡N bond means they are electrophilic at carbon and undergo many reactions analogous to aldehydes and ketones. They can be hydrolysed to acids, and reduced to amines. α-Hydrogen atoms are relatively acidic allowing the formation of delocalised anions and allowing alkylnitriles to be used as nucleophiles.
Acyl halides
Name =halocarbonyl- or -oyl halide
Example = butanoyl chloride or 3-bromocarbonylpropanoic acid
Examples:
Acyl halides are highly reactive or activated derivatives of carboxylic acids. The electronegative halide makes them electrophilic and they readily react with a range of nucleophiles in acyl substitution reactions to give acids, esters and amides. They are also used in the Friedel-Crafts acylation reaction.
Acid anhydrides
Name = -oic anhydride
Example = (symmetric) ethanoic anhydride or (non-symmetric) benzoic butanoic anhydride
Examples:
Acid anhydrides, both symmetric and non-symmetric (or mixed) are activated carboxylic acids akin to an acyl halide. They undergo the same acyl substitution reactions, reacting with nucleophiles, such as water to give acid, alcohols to give esters and amines to give amides.
Other Functional Groups
There are many other functional groups that you will meet as an undergraduate including hemiacetals (acetals with one hydroxy substituent replacing an alkoxy substituent), compounds containing phosphorus in various oxidation states (3 and 5) and with various numbers of P–O bonds (phosphines, phosphine oxides, phosphonic acids, phosphonates and phosphate esters), sulfonates and sulfonamides. This page is not meant to be comprehensive but just meant to give you a brief summary of the most common.