Stereochemical descriptors 3: Assigning a descriptor to a stereogenic plane
Introduction
This post continues the discussion of assigning stereochemical descriptors (or names) to stereogenic elements within molecules. Today, I’ll look at compounds that are said to be planar chiral (although, it has been pointed out that it is only organic chemists that like to break chirality down into these subcategories and we should all remember that compounds are either chiral or not, they are either superposable or not. Our models of bonding lead to stereoisomers and the categorization of chirality).
Anyway, it turns out that the assignment of a stereochemical descriptor to ‘planar chirality’ is a contentious subject (and one that confused me no end, until I worked out that there was more than one convention being used in the literature). It appears that cyclophanes (and similar molecules such as trans-cycloctene) and metallocenes are treated differently. To further complicate the matter, each of these classes of compound has more than one stereochemical naming convention. So, without further ado, let’s have a look at this mess …
Stereochemical Descriptor for Cyclophanes (based on a stereogenic centre)
Cyclophanes are treated as having a stereogenic plane (colloquially they are planar chiral) and are usually given the descriptor Rp/Sp or pR/pS, although it is recommended (but largely ignored) that they are described using helical chirality descriptors P and M (see next section). The configuration is based on a stereogenic centre and a tetrahedron. The diagram below shows how this tetrahedron is visualized. It should be noted that I don't know any cyclophane chemists (and I've talked to a few in my career) that use this method and a simplified method for determining the descriptor is given next.
The configuration of [2.2]paracyclophane based on a stereogenic centre or a tetrahedron. The plane, a & b, forms one edge of the tetrahedron and the out-of-plane atoms y & z are another. The priorities are a > b > y > z.
The long method is:
Step 1 - Identify the stereogenic plane. This is the plane with the most atoms in it.
Step 2 - Connect the in-plane reference atoms a & b, where a is the highest priority atom of the ring and b is the opposite atom from the branch point.
Step 3 - Assign the stereochemical descriptor as normal for a stereogenic centre with the priority order a > b > y >z. The descriptor is given a subscript p and is assigned to the ipso atom (orange dot below).
The molecule in this example, 4-bromo[2.2]paracyclophane, is Sp.
Method of assigning stereochemistry based on drawing a tetrahedron on the cyclophane.
The simplified method for assigning the stereochemical descriptor views the stereogenic plane from a pilot atom, and follows the rotation of the atoms towards the highest priority atom on the plane. A stepwise description of this process is:
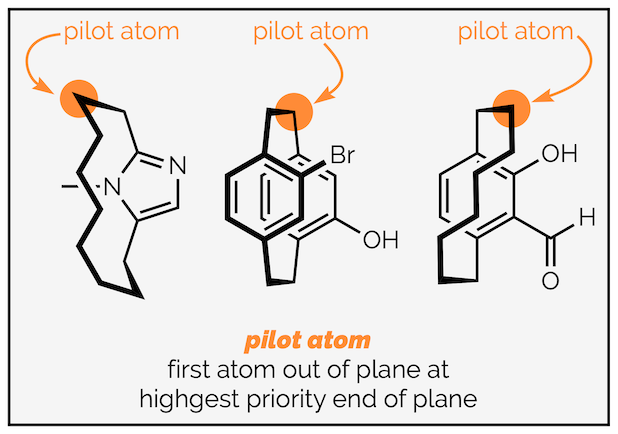
Step 1 - Identify the stereogenic plane - this will be the plane with the most atoms in it. This is frequently an aromatic ring. If there are two planes with the same number of atoms then the one with the highest priority atoms according to CIP rules is the stereogenic plane.
Step 2 - Identify the pilot atom - this is the atom directly attached, but not in, the stereogenic plane and closest to the end of the plane with the highest priority (CIP) atoms.
Step 3 - Starting from the pilot atom, view the plane and draw a line connecting the three highest priority adjacent atoms. This means starting at the atom next to the pilot and when you reach the ipso branch point following the atoms towards the highest priority atom.
- Step 4 - If the line connecting 1→2→3 is clockwise (right) when viewed from the pilot atom, the molecule is assigned Rp or pR planar chirality. Alternatively, if it is anticlockwise when viewed from the pilot atom, the molecule is Sp or pS.
Walking through an example is probably the best way to show these rules in action. Let’s determine the configuration of the cyclophane below:
Assign the configuration of this [2.2]paracyclophane.
Step 1 - identify the stereogenic plane. This is the plane with the greatest number of atoms (or highest priority atoms). In this case it is the top deck of the [2.2]paracyclophane due to the carboxylic acid taking precedent over the aldehyde.
The steps to determining the stereochemical descriptor for a planar chiral cyclophane.
Step 2 - identify the pilot atom. This is the first atom outside the plane at the end closest to the high priority atoms. It is the methylene group CH2 near the aldehyde.
Step 3 - number the atoms of the plane from the pilot atom towards the highest priority group. Number from the CH2 next to the pilot. This is atom 1. The ipso position is atom 2 and the carbon attached to the acid is atom 3.
Step 4 - Viewing the plane from the pilot atom, draw a line from 1 to 3 and the direction of the line determines the descriptor. If the line is clockwise the molecule has Rp stereochemistry. To show this clearly in this example, I have rotated the molecule so that the pilot atom is closer to you, the viewer. To be honest, as long as you remember which atom you have to view the plane from it is easier to reverse the answer than redraw the molecule (i.e. if you are looking down on the pilot atom reverse the answer).
Stereochemical Descriptor for Cyclophanes (based on a helix)
Planar chiral cyclophanes can also be described as a helix using either P or M. There are two interpretations of this, both give the same answer (whcih is nice, but not always the case in stereochemistry; see the metallocenes). The first follows an identical sequence to above, and simply uses a different letter as the descriptor. First, determine the pilot atom. Viewing the three atoms that move towards the highest priority group of the plane, draw a curve. If the curve (1→2→3) is clockwise (Rp), the descriptor is P. Alternatively, if it is anticlockwise it is M.
Describing a cyclophane as a helix.
Alternatively, P and M can be determined by:
Step 1 - Identify the plane as before.
Step 2 - Identify the pilot atom as before - this is the first out of plane atom closest to the high priority end of the plane.
Step 3 - Draw a Newman projection with the out-of-plane pilot atom at the front. If there is a negative angle when the pilot is rotated into the plane of the highest priority group the stereochemical descriptor is M. If the angle is positive then the descriptor is P.
This convention is reminiscent of the determination of the configuration of a conformer we discussed in the last post HERE.
Using a Newman projection to determine the helical descriptor of a cyclophane.
You will notice that P corresponds to Rp for planar chiral molecules, and M is Sp. This is the opposite to axially chiral molecules.
Metallocenes (recommended convention - a stereocentre)
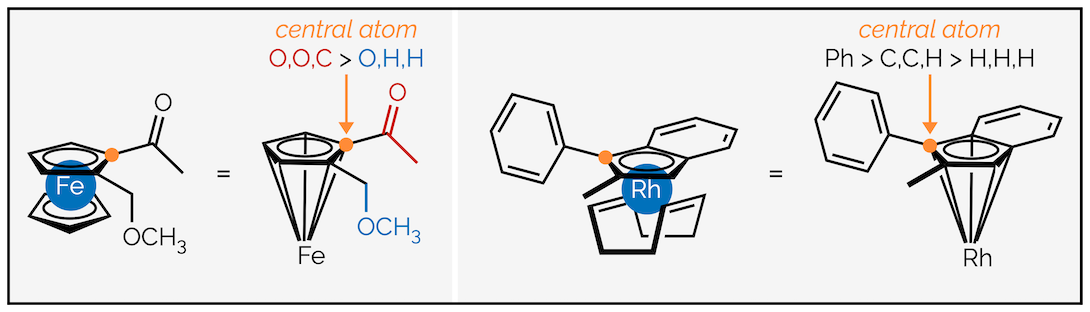
Metallocenes are treated differently to cyclophanes. The metal is considered to be σ bonded to the arene ring (just for naming purposes, obviously it isn’t). This allows a tetrahedron comprising of three atoms of the arene ring and the metal to be visualized. The atom at the centre of the tetrahedron is given a stereochemical descriptor in the normal manner for a stereogenic centre. To do this follow the guidelines below:
Step 1 - Identify the central atom of the tetrahedron. This is the highest priority atom of the arene ring as determined by the CIP rules. Frequently, it will be the atom with the highest ranked group attached. This is indicated by the orange dot below:
Step 2 - Rank the four groups attached to the central, orange, atom in the normal manner. The metal invariably has the highest priority.
Step 3 - Assign a descriptor to the stereocentre. Remember, the lowest priority group must point away from the viewer. Next draw a line connecting groups 1 to 3. If the line is clockwise the metallocene is given the descriptor R. If the line is anticlockwise it is S.
Metallocenes (older convention - a stereogenic plane - still common)
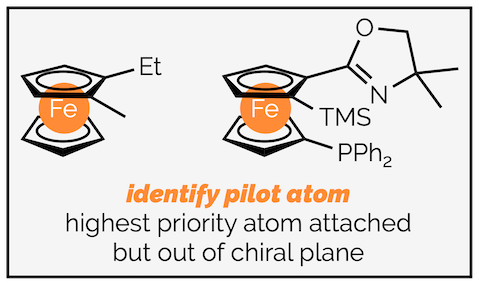
As is frequently observed in stereochemistry, there is more than one way of defining the chirality of metallocenes. There is an older system that is no longer recommended (not even by the scientist who initially proposed it) but is frequently encountered. It is based on describing the planar chirality and is similar to the method used for cyclophanes and helicity. Anyone working with planar chiral (ferrocene-based) ligands will meet this other system at some point. The method for defining the configuration is:
Step 1 - Identify the chiral plane. This will be the arene ring with the most substituents.
Step 2 - Identify the pilot atom. This will be the highest priority atom directly attached, but out of the chiral plane. This is most commonly the metal of the metallocene.
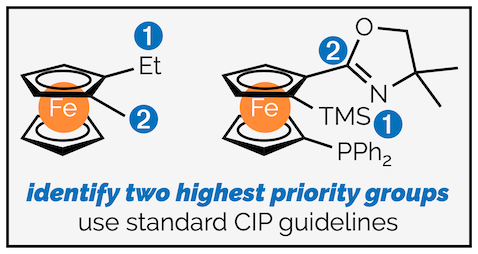
Step 3 - Choose the two groups on the chiral plane with the highest priorities.
- Step 4 - View the chiral plane from the opposite face to the pilot atom. If an arrow connecting the highest priority group to the next highest group is clockwise, the molecule is designated Rp. If it is anti-clockwise it is Sp.
The problem with these two systems is that they frequently give opposite stereochemical descriptor as can be seen if you look at the Josiphos family of ligands shown below. Using the recommended nomenclature based on central chirality, Josiphos is (R).
Assigning the stereochemical descriptor to the stereogenic plane of Josiphos using the recommended convention.
Using the planar chiral nomenclature it is (Sp). This is the nomenclature used by the companies selling this versatile, and useful, chiral ligand, even though Schlögl, who originally proposed this naming system, actually recommends using the newer system.
The older naming convention gives the opposite stereochemical descriptor.
Taking all of this into account, I would recommend always drawing planar chiral compounds (and I’m a cyclophane chemist) accurately rather relying on other chemists being able to interpret the stereochemical descriptor.
Conclusion
And you wonder why people hate nomenclature and particularly the naming of stereochemistry (actually, most people don’t wonder, it is fairly obvious)? It is complicated. It has been mired in many competing systems, and worse, it is not always clear which set of guidelines have been used. It is always best to draw the molecules.
The long promised worksheet with practice problems (including Noyori’s pre-catalyst) can now be found HERE.

![Stereogenic centre in [2.2]paracyclophane](https://images.squarespace-cdn.com/content/v1/62185f3b81809a6fd03ddbb5/4d5c2f02-ed5f-4c8e-8a0b-83447a747850/PC_tetrahedron.png)




![A disubstituted [2.2]paracyclophane](https://images.squarespace-cdn.com/content/v1/62185f3b81809a6fd03ddbb5/7eafdd48-d684-4231-8090-df21b06c5c5c/cyclocphane_example.png)