Acids & Bases
Introduction
Proton transfer is probably the most important reaction. It is a fundamental process found at the heart of many enzyme catalyzed reactions and without it there is no life. It is also a key learning tool. The reaction itself is simple, it is the transfer of a proton from one molecule, the acid, to another, the base, but what it can teach us about stability and reactivity can be applied to a host of organic transformations.
In this summary, I will be looking at the Brønsted-Lowry definition of acids and bases. An acid is proton donor and a base is proton acceptor. Many organic chemists (and organic textbooks) abbreviate acids to H+, a proton, and show each molecule attacking an isolated H+, but you should always be aware that H+ never exists in solution. A naked proton, H+, is simply too reactive and will always be associated with a base. Most often this molecule is the solvent, with the hydronioum ion H3O+, formed when an acid is in aqueous solution, being the most common example.
While seen in organic reaction mechanisms, H+ or a proton, does not exist and will always be associated with another molecule. If the reaction is in water then it will be a hydronium ion.
Acids and Bases
A typical acid base reaction is shown below with the deprotonation of acetic acid by hydroxide:
A typical acid base reaction.
In this reaction, acetic acid or CH3CO2H is the acid, it donates a proton, H+, to a base. It can be generalized as H–A. The base is hydroxide or HO– accepts the proton. It is normally generalised as B:. The reaction gives the acetate anion (carboxylate) CH3CO2–. This can accept a proton so is called the conjugate base. Conjugate means it is derived from H–A. It is normally generalised as A–. Water or H2O is the conjugate acid as it can donate a proton. It is called a conjugate acid as it is derived from the base B:. It is generalised as H–B+.
The general equation for a proton transfer or acid-base reaction is:
General form of the acid-base reaction.
Acid and bases must come in pairs and an acid will only behave as an acid if there is a base present to accept the proton. Pure hydrochloric acid is a non-acidic gas. The H–Cl bond is too strong to dissociate. Only one molecule in 10240 molecules of H–Cl dissociates. The latter is a ridiculously large number, to put it in perspective, there are thought to be only 1080 atoms in the universe.
Add a base, for example water, and hydrochloric acid is extremely acidic. The equilibrium constant (or acid dissociation constant), Ka, is 1 x 107. This means only one molecule in ten million has not dissociated and is still HCl.
The strength of acids and bases
It should come as no surprise that the strength of an acid (or a base) can be judged by inspecting the equilibrium constant (there was a reason that a simple organic chemist like myself covered equilibria in the last summary). For the general reaction below:
A generalized acid-base reaction.
A strong acid, H–A, will fully dissociate, with virtually all molecules losing their proton to the base. The acid is deprotonated and the base protonated. It favors the right-hand side of the reaction and will have a large equilibrium constant K. The acid is unstable or highly reactive. The conjugate base is far more stable and less reactive.
A strong acid fully dissociates and will favor the right-hand side. It will have a large equilibrium constant (> 10^2).
A weak acid, H–A, barely dissociates. It remains bonded to the proton (or protonated). The equilibrium favours the left-hand side and there will be a very small K, normally less than 10–2. The acid is stable and unreactive. Its conjugate base is unstable and reactive. The conjugate base readily reacts with a proton.
A strong acid does not dissociate much. The equilibrium favors the left-hand side.
The Strength of an acid measure by Ka or pKa
The importance of acids and bases in aqueous systems means it is common to measure their strengths in water and this leads to what is known as the acid dissociation constant (sometime known as the acid constant), Ka. This is a version of the equilibrium constant for the following general reaction. In this reaction the water acts as the base and the hydronium ion, H3O+, is the conjugate acid. As water is the bulk solvent, it is ignored (effectively it is hidden in Ka).
Acid-Base equilibrium in water.
Strong acids have large Ka and weak acids have small Ka.
The range of values of Ka is massive, even for compounds considered acids, never mind if you were to include compounds that are non-acidic (or basic). Mineral acids have Ka ranging from 102 to 109, while organic acids can are normally between 10–5 to 10–15. For instance, hydrochloric acid is a strong acid with a large Ka of 1 x 107. It is fully dissociated:
Hydrochloric acid has a large Ka and is effectively fully dissociated. The orange dot represents HCl while the black dots are the ions Cl– and H+.
Acetic acid CH3CO2H, a representative organic acid, has a Ka of only 1.74 x 10-5. This means roughly 17 molecules in a million have dissociated and the vast majority are still CH3CO2H.
Acetic acid is a weak, organic acid, with a small Ka. Only a small number of molecules have dissociated as represented by the black dots (the ions H+ and AcO–). The orange dots represent protonated acetic acid.
To make the numbers more ‘user-friendly’ pKa is used instead. This is a logarithmic scale that places common organic molecules on a range between approximately -14 and 53. The only catch is that pKa contains a negative term so the smaller the pKa value the larger Ka and the more acidic the compound.
The relationship between Ka and pKa.
A simplified example of the pKa scale is given below:
A simplified pKa scale. The boxes above give an idea of the scale of proton dissociation (black dot dissociated conjugate base - orange dot, the non-dissociated acid). Values are approximations (as there are multiple values available online).
From this, we can summarize:
- A strong acid, H–A, fully dissociates. H–A is unstable or reactive and A– is stable or unreactive. Ka is large, pKa is small (negative).
- A weak acid, H–A, partially dissociates. H–A is relatively stable or unreactive and A– is less stable or more reactive. Ka is small, pKa is large and positive.
What Controls the Strength of an Acid?
For the next discussion, I am going to ignore the solvent or base and just look at the acid. Remember, this is a simplification and that acids and bases must come in pairs.
The strength of an acid, HA, mostly depends on the stability of the conjugate base, A–. Dissociation of the acid is favoured if the conjugate base is stable and unreactive.
A strong acid HA:
- Has a low pKa.
- HA is disfavoured and it dissociates to a larger extent.
- HA is unstable and reactive.
- A– is favoured.
- A– is stable and unreactive.
- The stronger the acid the weaker the conjugate base, A–. The weaker a base A– is, the less likely it is to deprotonate another molecule.
Pictorial summary of a strong acid, which will have low (small or negative) pKa.
Hydroiodic acid, HI, has a pKa = -9.3. It is a strong acid that for all intents and purposes, fully dissociates. Its conjugate base, the iodide ion I-, is a weak base. It is relatively unreactive (in proton transfer reactions) and will deprotonate few, if any, compounds.
A weak acid HA:
- Has a high pKa.
- HA is favourable, it remains intact and doesn’t dissociate to a large extent.
- HA is stable and unreactive
- A- is unfavourable.
- A- is unstable and reactive
- The weaker the acid, the stronger the conjugate base A-. The stronger the conjugate base A- the more readily it will deprotonate another molecule.
Pictorial summary of a weak acid, which will have high pKa.
Methyllithium, CH3Li, can be thought of as CH3– (unless you are an inorganic chemist). It is the conjugate base of methane. It is a strong base, capable of ripping a proton from many compounds. Conversely, this means methane is a weak acid. It will not donate a proton. Its pKa reflects this and is high (48).
From this, you should see that a strong acid will have a weak conjugate base and a strong base will have a weak conjugate acid (but I'll hammer home this statement in the next section).
The next summary will show how we can use the structure of the conjugate base, A-, to predict the relative acidity of a compound HA.
Using pKa to Measure the Strength of a Base
I haven’t mentioned the base dissociation constant Kb or pKb. It exists and can be used to determine the strength of a base in exactly the same way pKa is used to measure the strength of an acid. Organic chemists tend not to use Kb or pKb. Instead pKa pulls double duty, serving as a scale of basicity as well as acidity. The previous section has already shown that there is a clear relationship between acids and bases:
- The stronger the acid HA (lower the pKa), the weaker the conjugate base A-.
- The stronger the base A- or :B, the weaker the conjugate acid AH (the higher the pKa (or BH+)).
It is the second statement that is important to an organic chemist. We use the strength of the conjugate acid of a base, as judged by pKa, as a proxy for the strength of a base. This is often quoted as pKaH, to remind us that we're looking at the conjugate acid ... but all too often it isn't.
This means that when an organic chemists makes a statement like:
“The pKa of butyllithum, BuLi, is 51, so it is a stronger base than sodium hydroxide NaOH with a pKa of 14.”
They most certainly do not mean that butyllithium is acting as an acid. The following reaction is garbage.
A meaningless reaction. Absolute fantasy but here to make a point (without any subtlely).
What a chemist is really saying is:
“The pKa of the conjugate acid of butyllithum BuLi is 51 (or pKaH = 51) and is higher than the pKa of the conjugate acid of hydroxide, which is 14 (or pKaH is 14), so BuLi is more basic. Or, the pKa of butane is 51 and the pKa of water is 14 so BuLi is more basic than HO-.”
In other words, we can predict the relative strengths of bases (the basicity) by comparing the pKa of the conjugate acids (the pKaH).
Using pKa to assess basicity.
A strong base B:
- Has a weak conjugate acid BH+ with a high pKa.
- BH+ is favoured and there is little dissociation.
- B: is unstable and reactive. It will deprotonate another compound.
A weak base B:
- Has a strong conjugate acid BH+ with a low pKa.
- BH+ is disfavoured and readily dissociates.
- B: is stable and unreactive. It will not deprotonate another compound.
The pH Scale
Most people are familiar with the pH scale even if they don’t understand where it came from. In this section I just want to give a brief outline of the salient features and how pH relates to the pKa of a molecule.
Simply put, pH is a measure of acidity in aqueous solutions. It is the related to the concentration of hydronium ion [H3O+] by the following equation:
To understand the pH scale it is helpful to look at the behavior of water. Water is odd. It is odd in many respects, just look at its boiling point compared to analogous compounds or the density of solid water. It is also odd as it is an amphoteric molecule. This means it is both an acid and a base. This means it will react with itself and the following equilibrium is established:
Water reacting as both an acid and a base. The small value of K shows the equilibrium favors the left-hand side.
The equilibrium constant, Kw, for this reaction is known as the water autoionization constant, the ion product of water or the dissociation constant (amongst a host of other names). It is small number, only one water molecule in over half a billion dissociates but it is incredibly important. It is important because it is a constant for all reactions involving H3O+ and HO– in water.
The pH scale comes from this equation. At neutrality, the concentration of the acid is the same as the base or [H3O+] = [HO–]. Substituting [H3O+] for [HO–] gives the following (the fine print: neutrality is pH = 7 at room temperature. Remember the equilibrium constant changes with temperature):
This is the centre of the scale. If the solution is acidic then [H3O+] > [HO–] and the pH < 7 (remember the negative log). Conversely, if the solution is basic [H3O+] < [HO–] and pH > 7. This leads to the following pH scale:
The pH scale.
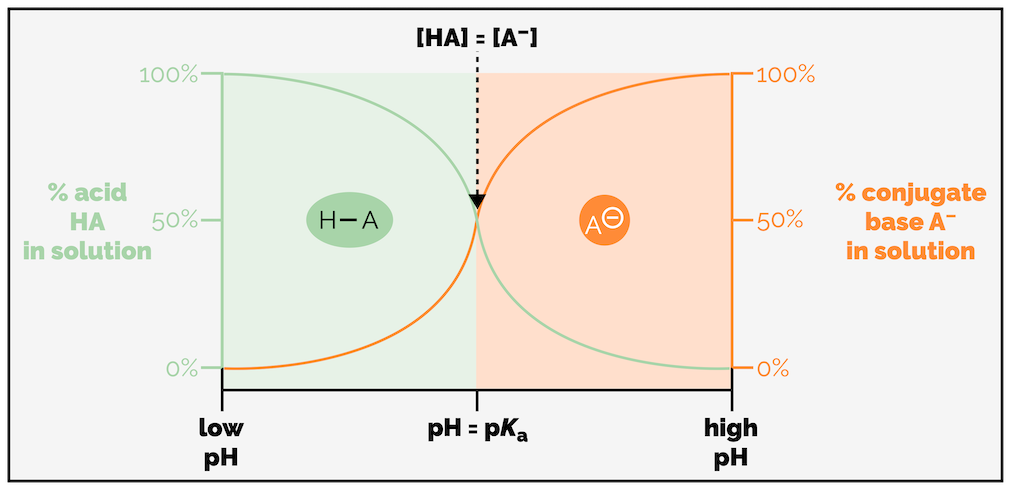
How does the pH of a solution relate to the pKa of a molecule? As you have seen, the strength of an acid can be judged by its pKa. The pKa of an acid can be defined as the pH at which an aqueous solution of the acid, H–A, contains 50% H–A and 50% A- or [HA] = [A-].
The relationship between the pKa of a molecule, the pH of a solution and whether a molecule is protonated or not. If the pH < pKa then the majority of the compound will be protonated. If the pH > pKa then the majority of the molecule will not be protonated.
If a molecule is in a solution that has a pH lower than the molecule’s pKa (pH < pKa) then the majority of the compound will exist in the protonated form. Should the pH be greater than the pKa (pH > pKa) then the molecule will mostly be deprotonated, in the form of the conjugate base.
Reactions always favor the weaker acid and base.
Looking at the reaction, you can compare the pKa values of the acid and the conjugate acid (pKaH), in this case acetic acid and water. The side of the reaction with the weaker acid will be favored. With the values being AcOH pKa = 4.8 and H2O pKa = 14, the right-hand side of the reaction is favored as water has a higher pKa and is the weaker acid.
Controlling Solubility
Organic molecules are rarely soluble in water due to their non-polar hydrocarbon backbone. Simplistically, like dissolves like, water is polar and the majority of organic compounds are mostly non-polar. Ionic salts are more soluble due to ion-dipole interactions. Adding a carboxylic acid or an amine to an organic compound makes it easy to form a salt through either deprotonation or protonation. This is one of the tricks used to make organic compounds more water soluble.
Fentanyl, a now infamous opioid painkiller, is barely water soluble at approximately 0.2 mg/ml. It is possible to protonate the amine functional group and form a salt, such as the ammonium citrate salt shown below. This has an increased solubility of 25 mg/ml this is the form of the drug commonly administered.
The salt, fentanyl citrate, is more water soluble than fentanyl.
Solubility can also have a profound effect on the activity of opioids such as fentanyl. Fentanyl has a pKa of 8.4 while its analogue alfentanil has a pKa of 6.5. Physiologic pH (pH of blood) is usually consider to be 7.4. If the pH of a solution is lower than the pKa of a compound then the compound is mostly protonated. If the pH is higher, the compound is mostly unprotonated or deprotonated. 90% of alfentanil is the amine in our blood while only 9% of fentanyl is, with 91% being an ammonium salt. This leads to alfentanil having a more rapid onset time (the non-polar form is absorbed quicker at the site of activity, it is more liophilic and crosses the appropriate membrane).
Protonation of amines at physiological pH. If the pKa of the conjugate acid (pKaH) is lower than the pH, the majority of the amine will be unprotonated. If it is higher, then the majority is protonated.
Acid-base extraction
Controlling protonation and solubility can be used to separate mixtures of compounds. A simple experiment performed at the start of many organic laboratory courses is acid-base extraction. At my home university, we separate a mixture of toluene, aniline and benzoic acid. The mixture is first reacted with aqueous sodium hydroxide. Only the benzoic acid is deprotonated. It is more acidic than the conjugate acid of hydroxide (pKa 4.2 versus pKa of water = 14) and the proton is transferred from one to the other. Remember the reaction proceeds to form the weaker acid. The salt is water soluble and dissolves in the aqueous phase.
Separation of a mixture of compounds by acid-base extraction.
Benzoic acid can be obtained by separating the aqueous layer and neutralizing the salt by the addition of a strong mineral acid such as hydrochloric acid. The benzoic acid is protonated and is no longer water soluble. The solid is filtered off. The aniline and toluene can be separated by reversing the process. Adding a strong acid to the mixture leads to the protonation of aniline, which then becomes more water soluble. The toluene can be separated. The aniline is then recovered by neutralization with sodium hydroxide, extraction with diethyl ether and evaporation.
Organic Reactions
A knowledge of acids and bases is invaluable when it comes to understanding many organic reactions. The factors that influence acidity, the stability of the conjugate base, are useful when thinking about leaving group ability and nucleophilicity, as well as proton transfer (somewhat obviously). As a result, one of the next summaries will discuss how you can predict relative acid strengths based on the structure of a molecule.
Conclusion
As always, this is summary is longer than the brief overview I had intended. The key points are:
- Acids and bases must come in pairs. There is a proton donor and proton acceptor.
- Acid-base reactions are an equilibrium. When an acid loses a proton it becomes a base called the conjugate base. When a base gains a proton it becomes the conjugate acid.
- The position of the equilibrium is normally given by pKa = -log Ka.
- A strong acid has a low pKa and a stable, unreactive, weak conjugate base.
- A strong base will have a weak conjugate acid with a high pKa (often written as pKaH) value.
- In a reaction a strong acid will always become a weaker base and a strong base will become a weaker acid. Reactions favor the formation of weaker acids and bases.
- pH measures acidity in water and can be useful for predicting protonation of molecules in solution.
A summary.