Le Chatelier’s Principle: Stressing Equilibria
… Or how to get a good yield of your product.
Stressing the equilibrium
Reactions are at equilibrium, and, as long as the temperature does not change, the equilibrium constant is just that, a constant. This means that any change you make to the reaction will be counteracted by the concentrations of the reactants changing to re-establish the equilibrium. This concept is eloquently expressed by Le Chatelier’s Principle:
“When a chemical system at equilibrium is disturbed, it responds by shifting the equilibrium composition in such a way to counteract the change.”
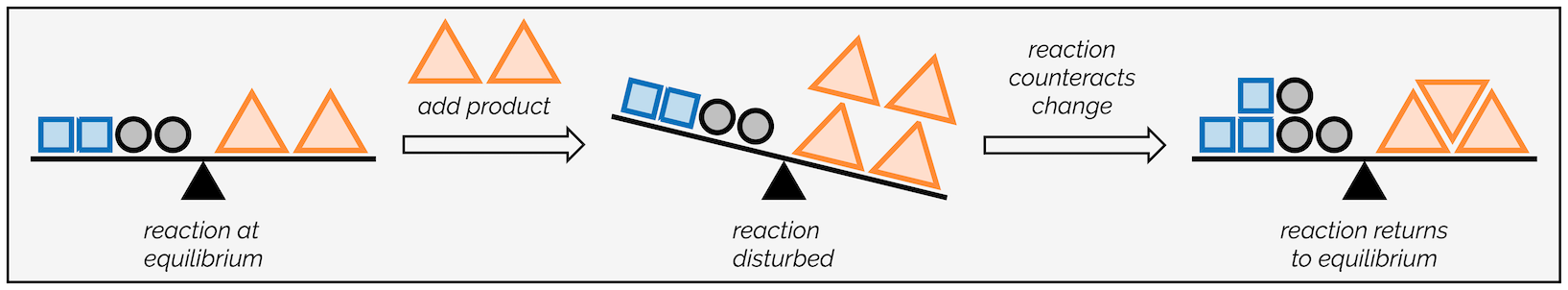
The simplest visualization of this is pretending that the reaction is a see-saw (or the wonderfully named ‘teeter-totter’ if movies are to be believed). When it is at equilibrium, it is balanced. If you add more product, the equilibrium is disrupted and the see-saw rocks in one direction. The reaction will regain balance by reacting some product and forming more starting material.
See-saw acting as an analogy for Le Chatelier’s Principle - if the equilibrium is perturbed, the reaction counters the change to restore balance.
For our discussion, the general reaction will be:
The equilibrium can be perturbed in a number of different ways:
Change of Concentration
If you add a reactant to the left-hand side of the equilibrium, the reaction will move to the right, increasing the concentration of product and decreasing the starting materials. The reverse is also possible. Add additional product and the reaction shifts to the left giving more of the reactants.
Increasing the concentration of a reactant on one side of the reaction will cause the equilibrium to shift in favor of the opposite side, increase the left side and the reaction shifts to the right.
The equilibrium constant, Kc, remains unchanged if you maintain a constant temperature. When more of one of the reactants is added, ratio of starting material to products becomes unbalanced. The reaction shifts to bring the ratio back to the original value of Kc. Adding starting material increases the denominator (below the line in a fraction), to maintain the value of Kc, the numerator (above the line) must increase, and this can only occur if more starting material reacts (and therefore decreases). The reaction shifts to the right.
The equilibrium constant must remain constant (if temperature unchanged), and reaction shifts to counteract any changes. Addition of starting material results in reaction moving forward.
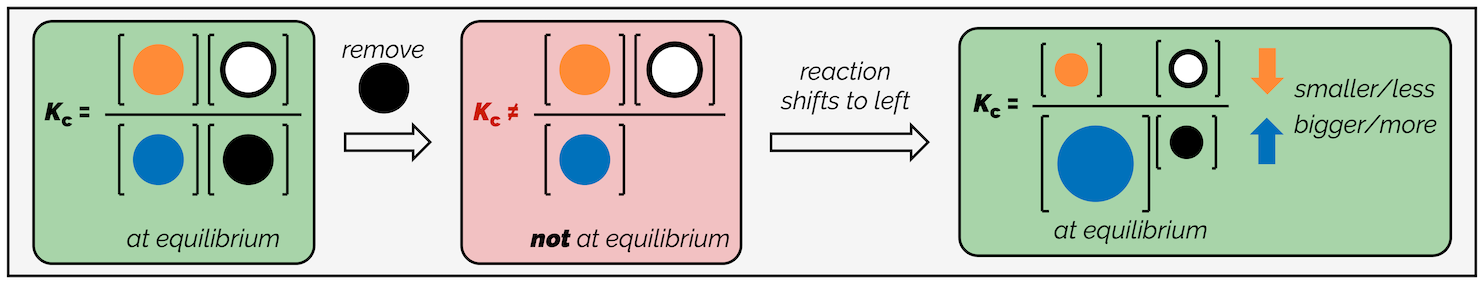
The same effect is caused by removing reactants. The reaction will counter this by consuming reagents on the other side of the reaction so that more of the removed substance can be formed. Removing starting material shifts the reaction to the left to replenish some of the missing reactant.
Decreasing the concentration of a reactant on one side of the reaction will cause the equilibrium to shift in favor of that side as the reaction tries to counter the change - remove reactant from the left and the reaction shifts to the left.
Again, looking at the equilibrium constant shows why the reaction must shift to the left in this example.
The equilibrium constant must remain constant at a fixed temperature. Removing starting materials decreases the denominator. To counter this the numerator must get smaller and more of the denominator (starting materials) must be formed.
Change of pressure
This only has an appreciable effect if at least one of the reactants is a gas; reactions involving only liquids or solids show no marked alteration in concentrations when the pressure changes. Before discussing the effect of changing the pressure, we need to make some assumptions and these are that the reaction is being performed in a sealed vessel and that the pressure is altered by changing the size of vessel (making the vessel larger causes a reduction in pressure while shrinking the vessel increases the pressure). The final assumptions is that the change in pressure does not effect the temperature.
If the pressure is increased, Le Chatelier’s Principle states that the reaction will counter this by shifting the equilibrium to favor the side with fewer molecules. The total pressure within the reaction vessel depends on the number of molecules of gas in the container. Pressure is related to the number of collisions between molecules and the container, the more molecules, the more collisions, and the greater the pressure. If you increase the pressure the equilibrium shifts to reduce this change or it shifts to reduce the number of moles of gas.
The example below shows the Haber process that converts nitrogen and hydrogen into ammonia. On the left-hand side there are four moles of gas, while on the right there is just two. Increasing the pressure results in the formation of more ammonia as the reaction counters the change by shifting to the side with fewer moles of gas.
Increasing the pressure favors the side of the reaction with fewer moles of gas - this counters the change by reducing pressure.
If pressure is decreased by expanding the size of the container, the reaction will try to cancel the change by shifting to the side that has more moles of gas. In the synthesis of phosgene, shown below, reducing pressure is countered by the reaction reversing to give more starting materials.
Decreasing pressure leads to the equilibrium shifting to the side with more moles of gas - this counters change by increasing pressure.
If the number of moles of gas is the same on both sides of the equilibrium then a change in pressure will have no effect. Shifting the equilibrium cannot counter the change in pressure.
Change of temperature
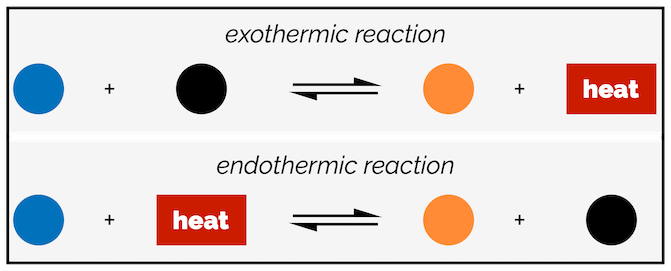
The effect of changing the temperature on the equilibrium depends on whether the reaction is exothermic or endothermic. The easiest way to visualize the influence of temperature is by adding heat or energy to the reaction equation. In an exothermic reaction, energy is released on formation of products. In other words, heat appears on the right-hand side of the equation. In an endothermic reaction, energy is absorbed in the reaction or it is one of the starting materials and appears on the left-hand side. If a reaction going from left to right, is exothermic, then the reverse reaction, from right to left, must be endothermic.
Simplified way of thinking about exothermic and endothermic reactions in equilibrium processes.
If you heat an exothermic reaction, you are effectively adding one of the products. The equilibrium will shift to the left to counteract the change and remove heat. The equilibrium is shifted by changing the value of Kc. Remember, Kc is related to the Gibbs free-energy change, which in turn is dependent on temperature, 𝚫G = 𝚫H – T𝚫S. This is the reason that we always quote the temperature when giving a value of Kc.
Again, if we look at the Haber process. It is an exothermic reaction, ammonia is more stable or has stronger bonds, than hydrogen and nitrogen. Energy is released on the formation of ammonia. Heating the reaction ‘adds’ a reactant to the right-hand side of the equilibrium. To counter this the equilibrium constant is reduced so that the starting materials become more favored and energy is absorbed.
Heating a reaction shifts the equilibrium to favor the endothermic reaction, while cooling a reaction will favor the side that releases heat, the exothermic reaction.
Now is a good point to remind you that our current discussion is only about which reactant is favored. It has nothing to do with the rate of reaction and how fast equilibrium is reached. The Haber process is a horribly complicated balancing act. The desired reaction is favored but is very slow. Far too slow to be useful. Heating the Haber reaction, increases the speed of the reaction but at the expense of yield. In the end, a compromise is reached and the reaction is performed at high temperature (400-450 °C) and high pressure in the presence of a catalyst. Even with this compromise, the reaction only proceeds with about 15% conversion to ammonia. The key is to recycle the starting materials continually.
And this leads us nicely to the discussion of how to get your desired product from an equilibrium reaction …
Using the equilibrium to get the the product you want
If all reactions are in a state of equilibrium, how do you get a good yield of the compound you want? You exploit Le Chatelier’s Principle and stress the equilibrium. The obvious way to do this is either to add an excess of one reactant or remove another. In order to maintain the equilibrium constant the reaction counter this stress by shifting in the desired direction and making more of the compound you want.
You can deliberately perturb the equilibrium in order to force the reaction to deliver the desired product.
Amongst the first reactions many chemists are taught are esterification, and the reverse process, ester hydrolysis. These are the two directions of an equilibrium process.
Esterification is the forward reaction while hydrolysis is the reverse or backward reaction. The equilibrium constant is approximately 1 (possibly as high as 4).
The equilibrium constant for this reaction is close to 1.* This means that, left to its own devices, either of these reactions would give a 50:50 mixture of ester + water and carboxylic acid + alcohol. This is not particularly useful. How could you force the reaction to give you a high yield of ester?
*I have included water in the equilibrium expression as I have assumed that it is not the bulk solvent (this is almost certainly true for esterification but might not be as save an assumption with hydrolysis).
There are two simple solutions that depend on the equilibrium constant remaining constant (so they don’t involve changing the temperature). The first is adding more alcohol. If you add a reactant, the concentration of the other reactants must change to maintain the original ratio, the equilibrium constant. Add more alcohol, and the concentration of the acid will decrease while the concentration of both the ester and water will increase.
Adding alcohol causes the concentration of carboxylic acid to decrease and the concentrations of ester and water to increase in order to maintain a Kc close to 1.
Alternatively, removing one of the products from the reaction will result in the concentration of the other increasing. Remove water, and, in order to maintain a Kc of approximately 1, the concentration of the ester must increase along with the concentrations of the starting materials decreasing. Both effects shift the reaction to the right and give you more of the desired product (and such a change is easily achieved in a laboratory setting with the use of Dean-Stark apparatus (just in case my second years are reading this)).
Conclusion
The equilibrium constant at any given temperature is just that, a constant. If you change the concentration of any of the compounds or the pressure of the system, the reaction will proceed in the direction that counters the stress you applied to the system until it returns to equilibrium. You can use this to force the reaction to give you the compound you want.
Controlling the equilibrium using Le Chatelier’s Principle.
Practice questions will appear HERE.