Nucleophilic Acyl Substitution
Introduction
Acyl substitution involves the transfer of RC(O) from one reactant to another, as shown below:
Nucleophilic acyl substitution is a reversible reaction.
At the heart of this reaction are the same basic ideas that have explained all additions to the carbonyl group:
The carbonyl bond is reactive due to the polarization of the bond that makes the carbon atom electrophilic.
The carbonyl bond is strong (it is a double bond and the polarization adds ionic character) and will reform if it can.
Key to understanding acyl substitution is the leaving group attached to the carbonyl group. This allows the carbonyl group to reform. This means that a substitution reaction is now possible as there can be an addition and elimination reaction occurring.
Carboxylic Acid Derivatives
Nucleophilic acyl substitution is the reaction of a nucleophile with a carboxylic acid derivative or acyl group. This is a carbonyl group attached to a leaving group. They have the general formula given below:
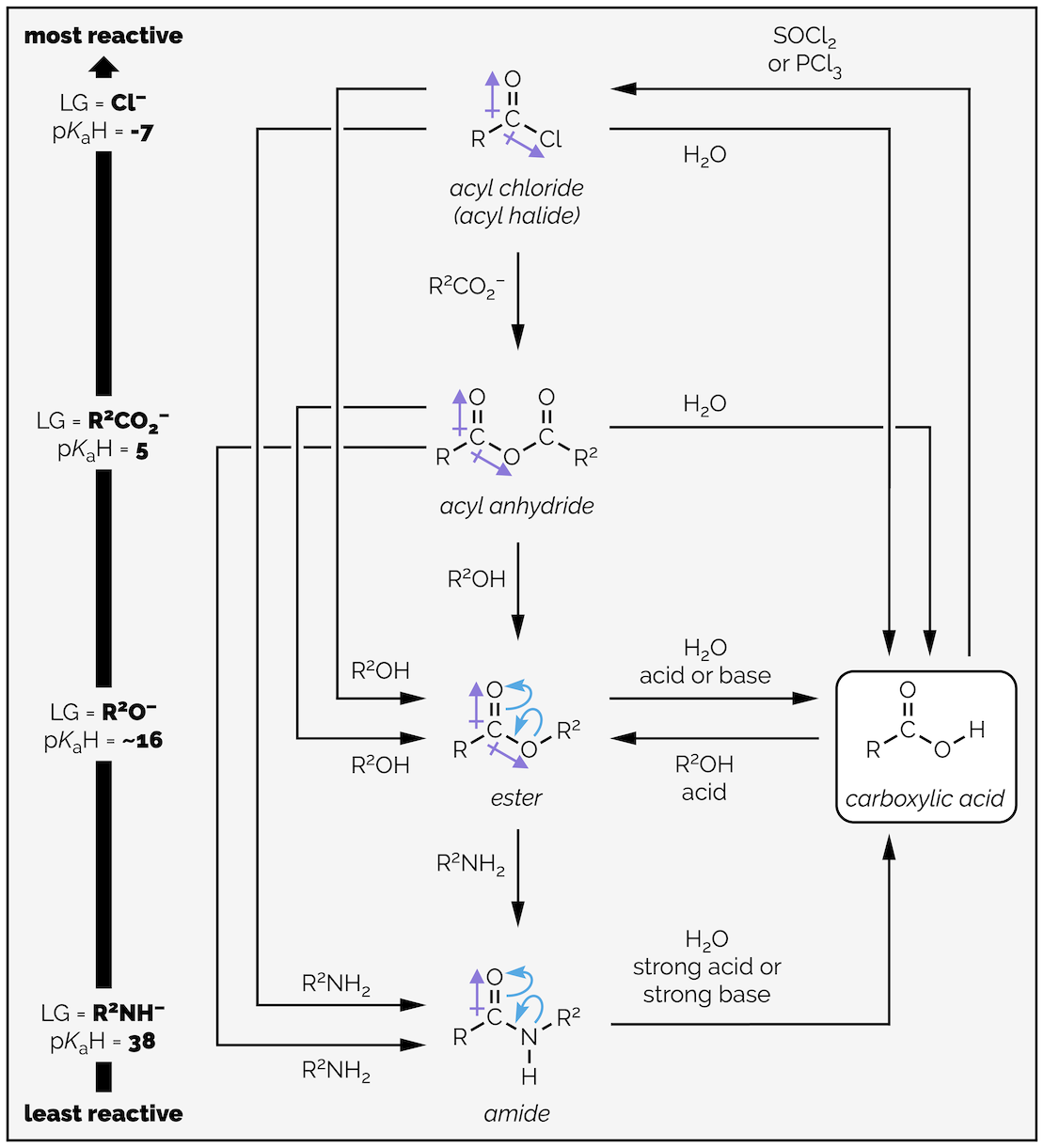
Carboxylic acid derivatives and their relative reactivity, which to large extent is based on leaving group ability. This, in turn, is related to base strength and the pKa of the conjugate acid.
The chemistry of carboxylic acid derivatives can be understood if you consider that each one comprises of a carbonyl group attached to a leaving group. The carbonyl group is highly polarized, making the carbon atom electrophilic, but the carbonyl group is also strong. This means it wants to reform if possible. The presence of the leaving group means it always has a route to reform. A leaving group is an electronegative atom or group that will take two electrons away from the molecule. The best leaving groups form neutral molecules or stable anions. They are weak bases or have a strong conjugate acid (pKaH is low).
Acyl chlorides are the most reactive carboxylic acid derivatives. The chlorine atom is highly electronegative and this polarizes another bond attached to the carbon making it even more electrophilic. The chloride anion is a good leaving group being a stable anion (pKaH = -7). Both factors make the acyl chloride reactive.
The reactivity of various carboxylic acid derivatives (and aldehydes and ketones as a sort of baseline) can be explained by either balancing polarization and delocalization or leaving group ability.
Anhydrides are less reactive than acyl chlorides even though oxygen is more electronegative than chlorine. There are now two competing effects. Polarization of the σ bond should increase electrophilicity but this is countered by delocalization of the oxygen lone pair. This increases electron density on the carbon, and reduces electrophilicity. This isn't an issue for chlorine due to the difference in size between chlorine and carbon, which leads to more orbital overlap and reduced delocalization. A second argument for the reduced reactivity is that the carboxylate anion leaving group is less stable (a stronger base than the chloride anion). The carboxylate anion is stabilized by delocalization and is still a good leaving group making anhydrides reactive.
Esters are less reactive than anhydrides. Delocalization of the oxygen lone pair lowers the electrophilicity of the carbonyl group. In the anhydride the lone pair could be spread over two carbonyl groups so had less of an impact. Furthermore, the alkoxide leaving group formed in the reaction of an ester is less stable than a carboxylate as it does not benefit from delocalization. With a poorer leaving group you have a less reactive carboxylic acid derivative.
Finally, the least reactive derivatives are the amides. The nitrogen is less electronegative than oxygen or chlorine and delocalization of the nitrogen lone pair of electrons plays a more substantial role. The amide anion is a poor leaving group being a reasonably strong base. Both reasons lead to the reduced reactivity.
You will have noticed that I haven't included carboxylic acids themselves in either list of reactivities. They are a special case. From the discussion above, you should be able to predict that their reactivity will be similar to that of an ester (R2 is simply a proton instead of an alkyl group), and that they are more reactive than an amide. The hydroxide anion is a marginally better leaving group than an alkoxide anion and a considerably better leaving group than an amide anion (sorry, the term amide refers to both RC(O)NHR2 and R2NH-).
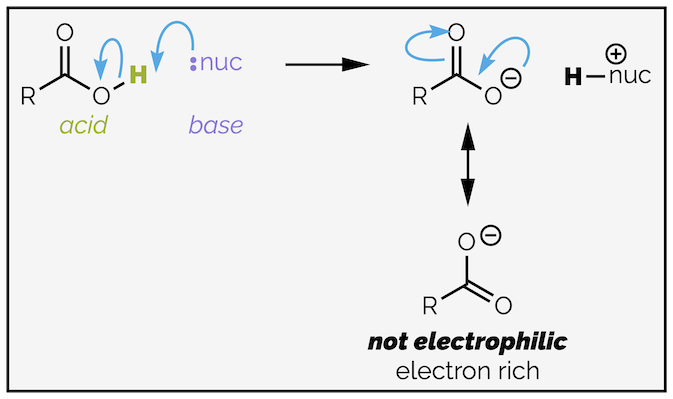
The problem with carboxylic acids is the acidic proton. It can react with a nucleophile (or base) instead of the carbonyl group. Proton transfers are fast reactions. The product of proton transfer is a carboxylate anion, which is no longer electrophilic being an electron rich species.
Carboxylic acids have an acidic proton than can interfere in nucleophilic acyl substitution reactions. The nucleophile (or base) can deprotonate the acid resulting in the formation of a carboxylate anion. Carboxylate anions are the least reactive carboxylic acid derivatives as they are electron rich.
General Reaction Mechanism
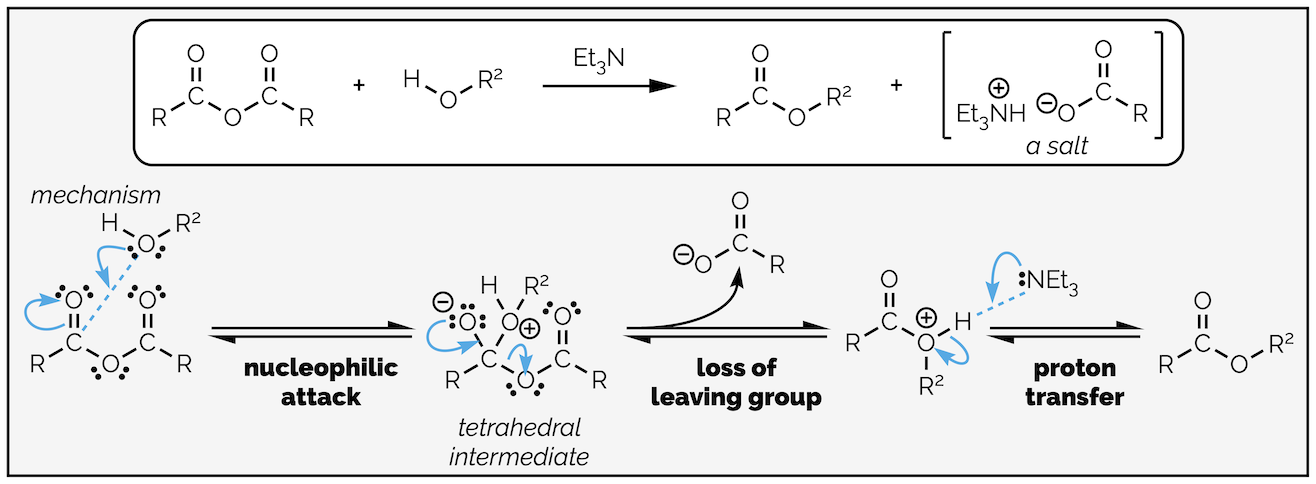
Nucleophilic acyl substitution of a carboxylic acid derivative always involves at least two steps, nucleophilic addition to the carbonyl group and loss of a leaving group (known as elimination). Depending on which derivative is reacting and the nature of the nucleophile there will be one, or more proton transfer steps. The position of the proton transfers in the reaction sequence will change with reaction conditions.
The simplest example is addition of an anionic nucleophile to a carboxylic acid derivative as shown below:
Nucleophilic acyl substitution with an anion nucleophilic occurs by an addition-elimination mechanism or written out in full, nucleophilic addition followed by loss of a leaving group.
The nucleophile attacks the electrophilic carbon atom creating a new σ bond at the expense of the weak π bond with the electrons flowing towards the electronegative oxygen atom. This gives the tetrahedral intermediate. The carbonyl moiety is a strong bond being two covalent bonds with ionic character. Given a chance it will reform. As carboxylic acid derivatives have at least one leaving group on them, the tetrahedral intermediate collapses with loss of the leaving group or elimination. This gives the product.
If the reaction involves a neutral nucleophile then there will be a proton transfer step in order to form a neutral final product.
Nucleophilic acyl substitution with a non-charged nucleophile. The reaction still involves nucleophilic addition and loss of the leaving group but it now has a proton transfer.
The reaction is the same as before, it starts with nucleophilic addition to the carbonyl group. This gives a tetrahedral intermediate. The best leaving group is likely to be the charged nucleophile as this would result in a neutral product being kicked out. This gives the starting materials back, and as I’ll discuss below means these reactions are reversible. Alternatively, there can be a proton transfer to form a new tetrahedral intermediate. Once this has occurred the reaction again follows the first example and the tetrahedral intermediate collapses kicking out the best leaving group and giving a new product. The reaction is the same, there is just a proton transfer step.
Protonating the carbonyl group makes it more electrophilic, and this can accelerate the reaction. The reaction is now acid catalyzed. The mechanism is more or less identical to before, and contains both nucleophilic addition and loss of a leaving group but now there are a number of additional proton transfer steps.
Acid-catalyzed nucleophilic acyl substitution with a non-charged nucleophile (the nucleophile can't be an anion otherwise it would be destroyed by the acid). The reaction still involves nucleophilic addition and loss of the leaving group but it now there are four proton transfer steps.
The first step is protonation of the carbonyl group. This increases the polarization and makes the bond more reactive, more electrophilic. Nucleophilic attack as before leads to a tetrahedral intermediate. A series of proton transfer steps leads to protonation of the leaving group, which is then eliminated to reform the carbonyl group in the form of an oxonium ion. Another proton transfer effectively regenerates the catalyst and gives the product.
In all these general (meaning I haven't defined the nucleophile or the leaving group) mechanisms, you will see that I have used double arrows to indicate that each step is reversible, and that an equilibrium will be established. This is due to the tetrahedral intermediate. As stated, the carbonyl wants to reform and the tetrahedral intermediate will collapse to give a C=O double bond if there is a leaving group present. What you need to remember is that the incoming nucleophile (especially when it is neutral) is also a good leaving group so that reaction could return to starting materials. This means the position of the equilibrium is determined by the relative stability of the leaving group compared to the nucleophile. The reaction will favor whichever is more stable. Or, if you want to form one substrate predominantly, you will need to perturb the equilibrium to force it in the direction you want. This can be achieved by adding or removing reactants, or, as you shall see, by the correct choice of reagent so that you make one of the steps irreversible.
Nucleophilic acyl substitution is reversible and an equilibrium will be established that favors formation of the more stable leaving group unless perturbed.
Reactions of Carboxylic Acids
Nucleophilic acyl substitution of a carboxylic acid can be difficult as the acidic proton interferes. It is more common to convert the hydroxyl group, OH, into a good leaving group first. Carboxylic acids can be converted to acyl chlorides by reaction with thionyl chloride or phosphorus trichloride (and other reagents). Both methods convert the hydroxyl group into a good leaving group before the chloride is added:
Carboxylic acids can be converted into acyl chlorides by reaction with thionyl chloride (SOCl2). The reaction follows the standard acyl substitution reaction with a nucleophilic addition step and a loss of a leaving group.
The mechanism follows the standard pattern having both a nucleophilic addition step and the loss of a leaving group from a tetrahedral intermediate. There is one additional step, the activation of an oxygen atoms as a leaving group (converting OH, a poor leaving group into a good leaving group). In the first step, an oxygen lone pair attacks the highly electrophilic thionyl chloride. Thionyl chloride is a good electrophile as there are three electronegative atoms on the central sulfur atom. The chloride anion that is formed in this step then acts as a nucleophile and attacks the reactive oxonium ion to give a tetrahedral intermediate. Reformation of the carbonyl bond results in the leaving group being kicked out. The leaving group is SO2, a gas, which bubbles out of the solution making this step irreversible (it is no longer in the reaction mixture), and this forces the reaction to completion.
This version of the mechanism is acceptable (or I wouldn’t have drawn it). It is the short form of the reaction. There is a longer version involving numerous proton transfers. This longer mechanism is probably closer to reality as it permits better orbital overlap when the bonds are made and broken, but I’m pretty sure you will not mind having a shorter version!
The most common student mistake is to think that acyl chlorides can be made from the reaction of a carboxylic acid and hydrochloric acid. They can’t. You can see why this is the case if you inspect the various leaving groups.
Acyl chlorides cannot be made by reacting carboxylic acids with hydrochloric acid.
Once the chloride ion has added to the carbonyl group, a tetrahedral intermediate is formed. This will collapse to reform the carbonyl group and kick out or eliminate a leaving group. The choice of leaving groups is the chloride anion, the hydroxide anion or, after a series of proton transfers, water. The chloride anion is the weakest base (lowest pKaH) and best leaving group. The reaction favors the starting materials and this is not a method for the formation of acyl chlorides.
One of the first reactions I was ever taught (and one of the first I ever did) is esterification by combining a carboxylic acid and an alcohol in the presence of an acid catalyst. The reaction follows the standard acid-catalyzed acyl substitution mechanism.
The acid-catalyzed esterification or formation of an ester from the reaction of a carboxylic acid and an alcohol.
The acid catalyst speeds up the reaction in two ways, protonation of the carbonyl group to form the oxonium ion makes the carbonyl group more electrophilic. This helps the alcohol, a weak nucleophile, attack the C=O double bond. Secondly, the acid protonates the hydroxyl group to make it into a better leaving group. The hydroxide anion is not a good leaving group but water is. Both factors encourage the reaction to proceed.
The mechanism involves a proton transfer to create the good electrophile. Then there is the standard nucleophilic attack to give the tetrahedral intermediate. A series of proton transfers make a good leaving group so that we can have the second important step, the loss of the leaving group with reformation of the carbonyl group. There is a final proton transfer that gives the proton and regenerates the acid catalyst.
The reaction is reversible. The two potential leaving groups, water or an alcohol, are equally good, they have similar basicities (between 14 & 17). This means that it is necessary to force the reaction in the desired direction. If you want the ester either add an excess of alcohol or remove the water. If you want to convert an ester into the carboxylic acid, a reaction called hydrolysis, then add an excess of water.
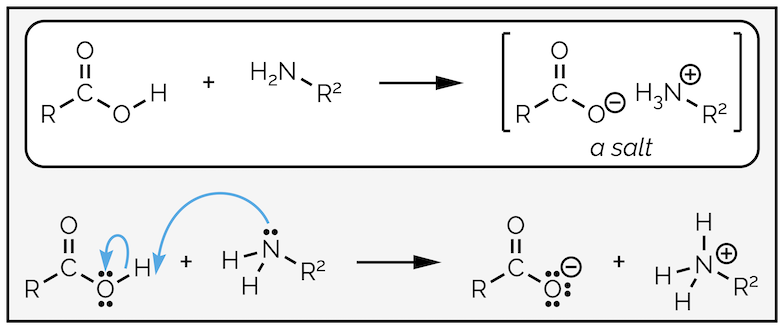
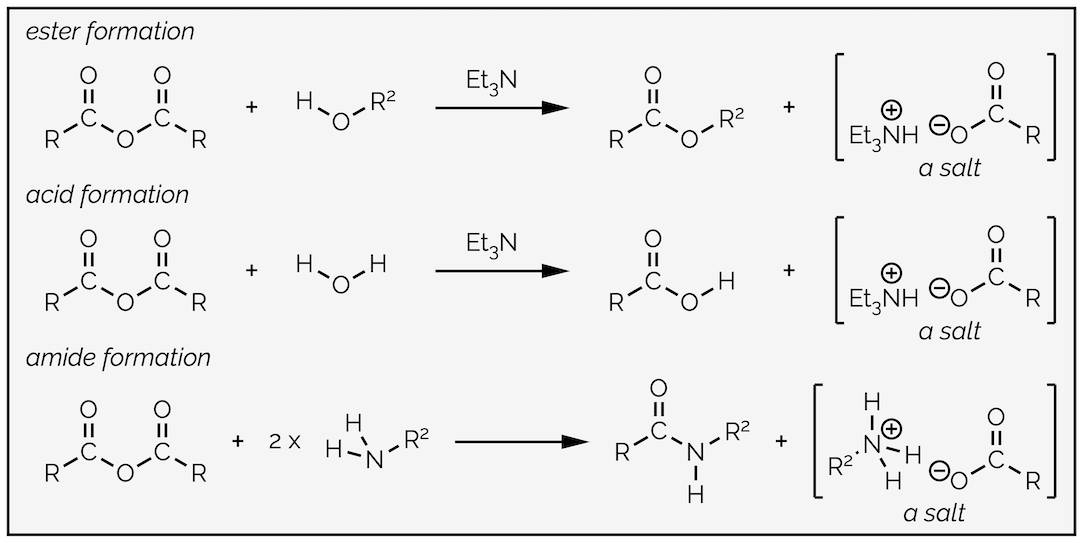
Carboxylic acids do not react under basic conditions. This means they will not react with amines to give an amide (actually this is a slight lie, they will but it requires high temperatures, normally in excess of 150 °C). The reaction of a carboxylic acid and an amine at room temperature will give a salt (this is a popular exam question with examiners).
Amines behave as bases not nucleophiles when they react with carboxylic acids.
Amides can be prepared indirectly from any of the more reactive carboxylic acid derivatives (acyl chlorides, anhydrides and esters). In fact, there are many, many different ways of forming amides from carboxylic acids using a whole host of different reagents (it’s like amides, peptides, and proteins are important in biology or something). While there are many named reagents (it is an alphabet soup of acronyms or abbreviations (to steal someone else’s ‘joke’)) they all work in the same manner, they activate the hydroxyl group, making it into a good leaving group. This means all these methods occur by the same standard acyl substitution with two steps common to every mechanism, nucleophilic addition and loss of a leaving group. The difference is either in how the oxygen is activated or in the proton transfer steps.
The Reaction of Acyl Chlorides
Acyl chlorides are the most reactive of the carboxylic acid derivatives. They can be converted into any of the others by the simple general mechanism you have worked through above. Acyl chlorides react with water, a reaction known as hydrolysis, to give carboxylic acids. The reaction involves nucleophilic addition followed by loss of a leaving group and then proton transfer.
Hydrolysis of an acid chloride to a carboxylic acid is another example of nucleophilic acyl substitution. The water is the nucleophile and attacks the carbonyl group before the chloride is eliminated.
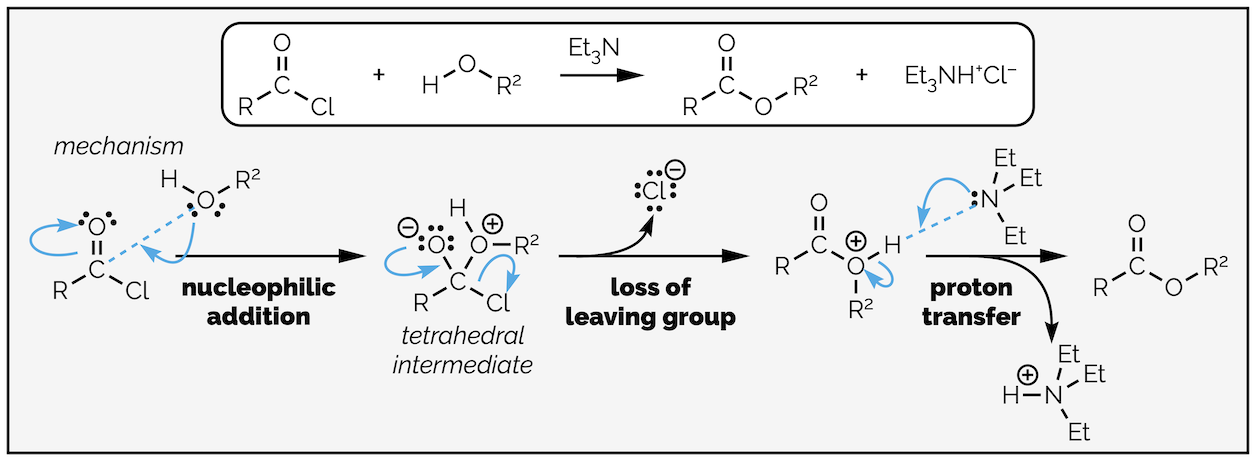
Replacing water with an alcohol provides a route to esters. The mechanism is exactly the same (you are going to get bored of me typing this). In these reactions you will notice that the by-product is hydrochloric acid (HCl), a strong acid. Strong acids can react with many different functional groups and destroy your molecule. To avoid this, it is common to add a base to the reaction. The base is not strong enough to deprotonate the nucleophile, it is present to mop up the acid formed in the reaction. This is shown in the esterfication below.
Esterification by the reaction of an acyl chloride with an alcohol. In this example a base, triethylamine, has been added to neutralize the acid formed in the reaction so that it does not damage the product.
By now you should be getting the hang of this reaction. The nucleophilic alcohol attacks the electrophilic carbonyl group. This gives the tetrahedral intermediate, which collapses to reform the carbonyl group and kicks out the leaving the group. The base, triethylamine, then removes a proton in a proton transfer step. As with most of the summaries, there is a single reaction that can be applied to different transformations. Organic chemistry isn’t a huge number of reactions that need memorizing, it is a few principles applied over and over again!
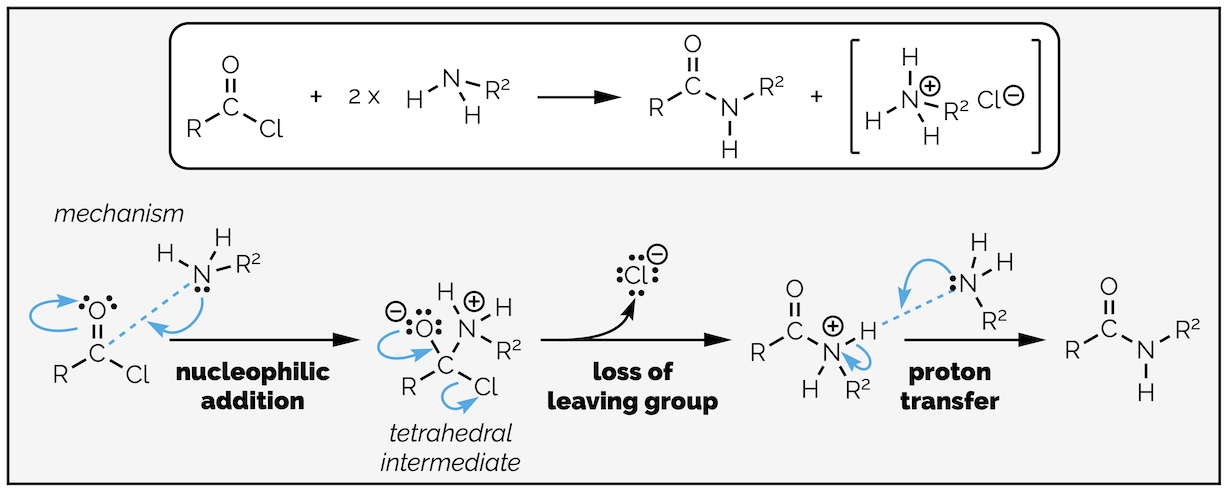
Amides can be made from acyl chlorides by exactly the same reaction and mechanism as the previous examples just replacing the nucleophile with an amine. It is nucleophilic addition followed by loss of the leaving group and then a proton transfer. It is not necessary to add a base to this reaction, the amine can acts as base, but you must remember to add two equivalents of amine. If you don’t, protonation of the amine forms a salt that is no longer nucleophilic and you only get 50% yield. If the amine is precious then you can add a base, just make sure it is a strong base than your amine!
Formation of an amide from an acyl chloride. Follows the same mechanism as all the reactions of an acyl chloride. There are three steps, nucleophilic addition, loss of a leaving group and a proton transfer.
Reactions favor the formation of more stable, less reactive compounds. As acyl chlorides are the most reactive of the carboxylic acid derivatives, they can be transformed into any of the other derivatives.
Reactions of Anhydrides
Anhydrides are less reactive than acid chlorides but are more reactive than esters, acids, and amides. They can be made by reacting an acid chloride with a carboxylate anion as the nucleophile. This follows the same reaction mechanism as above. The chloride is a better leaving group than a carboxylate anion (compare the pKaH of -7 and ~ 5 respectively) so the reaction favors formation of the anhydride. If the two sides of the anhydride are not the same (R ≠ R2) the molecules is called a mixed anhydride (and you have to start worrying about what side will react first).
One method to prepare (mixed) anhydrides.
The mechanism for all their reactions is the same as the acid chlorides. It involves nucleophilic addition to form the tetrahedral intermediate followed by loss of a leaving group and proton transfer. Reaction with an alcohol will give an ester, reaction with water an acid, and reaction with an amine will give an amide.
The reaction of an anhydride with an alcohol to give an ester. This follows the standard nucleophilic acyl substitution reaction. Reactions of water and amines are exactly the same.
Reactions of anhydrides to give a variety of different products.
Reaction of Esters
Esters and carboxylic acids have a similar reactivity in terms of nucleophilic acyl substitution, but esters have the advantage that they do not have an acidic proton. This means they react under both acidic and basic conditions. They can be hydrolysed to acids or converted to amides.
Conversion of an ester to an amide follows the standard mechanism. It is a favorable reaction as you are moving down the reactivity scale with amides being less reactive than esters, or, to think about the leaving group on the common tetrahedral intermediate, alkoxides/alcohols are better leaving groups than amides/amines and so formation of the amine is favored. Interestingly, textbooks vary on the order of proton transfer (do you kick out an alkoxide or an alcohol?). I don't think it makes any difference, both mechanisms rely on a disfavored step occurring before you get to the favored product. What do I mean by this? Either an alkoxide has to be eliminated in the presence of an ammonium species, meaning a poor leaving group is kicked out, or you have to protonate an ether-like oxygen in the presence of the more basic amine. Neither process is favored but the whole point of an equilibrium is that all processes occur, they’re just reversible. It is what happens next that drags the reaction towards the more stable products. Here is one of the mechanisms.
Reaction of an ester with an amine to give an amide. In this version of the mechanism, elimination occurs before proton transfer.
As stated, the mechanism is the same as before. The amine acts as a nucleophile and attacks the carbonyl group to give a tetrahedral intermediate. Most of the time the ammonium ion is kicked out to reform the starting material but a small amount of the tetrahedral intermediate collapses in the other direction to eliminate the alkoxide. This could attack the carbonyl group again but proton transfer is much faster and the amide is formed. Amides are considerably less electrophilic than esters and alcohols are less nucleophilic than amines. This means the reaction is moving towards the favored products and eventually goes to completion.
Acid catalyzed hydrolysis of an ester to a carboxylic acid is the reverse of esterification covered earlier. It is an equilibrium reaction and it is necessary to drive the reaction in the direction you require. In terms of hydrolysis, this means adding an excess of water.
Acid catalyzed hydrolysis of an ester to give a carboxylic acid is the reverse of esterification. The mechanism is exactly the same.
The mechanism of acid catalyzed hydrolysis is exactly the same as esterification. It involves proton transfer to activate the carbonyl group, followed by nucleophilic addition to give the tetrahedral intermediate. A series of proton transfers sets up collapse of the tetrahedral intermediate with elimination of alcohol as the leaving group. A final proton transfer gives the carboxylic acid and regenerates the acid catalyst.
Base-mediated hydrolysis of an ester is different. This is no longer a reversible process and the reaction goes to completion (all the starting ester should be consumed). The mechanism still involves the same steps.
Base-mediated hydrolysis of an ester is an irreversible process. The carboxylic acid is only an intermediate and it is deprotonated in an irreversible step to give a carboxylate anion. This species is not electrophilic so the reverse reaction is no longer possible. The carboxylic acid can only be isolated by neutralizing the reaction.
The base mediated hydrolysis of an ester starts in the same way as all the previous reactions. The nucleophilic hydroxide anion participates in nucleophilic attack to give a tetrahedral intermediate. The carbonyl bond reforms by kicking out a leaving group. The hydroxide is actually the better leaving group but occasionally the alkoxide is eliminated. In the theory, the resulting carboxylic acid could undergo nucleophilic attack by the alkoxide to go back to the tetrahedral intermediate but it doesn’t. Proton transfer is a faster reaction and the alkoxide acts as a base and deprotonates the acid to give a carboxylate anion. The carboxylate is not electrophilic. It is electron rich and will not react with the alkoxide to reverse the reaction. At this point, the reaction is irreversible. The carboxylate will just sit there until you add acid to neutralize the reaction. This is the only point at which you can isolate the carboxylic acid. This is useful.
The base-mediated hydrolysis of an ester is known as saponification. It is the chemistry required to make soap from triglycerides (lipids).
The Reaction of Amides
Amides are the least reactive of the (neutral) carboxylic acid derivatives. The nitrogen is less electronegative than oxygen or a halide so the bond is less polarized and delocalization of the lone pair further diminishes the electrophilicity of the carbon atom. This means the reactions of amides are limited with hydrolysis being the only substitution reaction of importance. This can either be achieved with enzymes (it is vital for all living systems) or harsh conditions in the laboratory. While harsh conditions are required, it can be promoted by either acid or base and the reaction is irreversible (due to further reactions of the products).
The acid-promoted reaction follows the standard mechanism with activation of the electrophile by protonation of the carbonyl group being the first step.
Acid-mediated amide hydrolysis follows the standard nucleophilic acyl substitution reaction with additional proton transfer steps. The reaction is irreversible due to the protonation of the amine, which makes it non-electrophilic. This also means that at least one equivalent of acid is required for the reaction to proceed fully.
The first step is a proton transfer to form the activated electrophile or oxonium ion. The oxygen is more basic than the nitrogen due to the delocalization of the nitrogen lone pair in an amide. The increased polarization of the carbonyl by protonation allows the nucleophilic addition of water. The nitrogen in the tetrahedral intermediate is now an amine, delocalization is no longer possible so it is basic. A series of proton transfers result in it becoming a good leaving group. The carbonyl bond reforms and the amine is eliminated. All steps up to this point have been reversible but now the amine is protonated under the acidic conditions. Once protonated it is no longer a nucleophile and the reaction cannot go in reverse. This means that the reaction slowly proceeds to give the desired product of hydrolysis.
While amides are poor electrophiles, nucleophiles will attack them under sufficiently vigorous reaction conditions. Heating the amide in a solution of hydroxide allows nucleophilic attack. The mechanism is, unsurprisingly, the same as all the other examples in this summary. The reaction is irreversible due to the carboxylic acid being deprotonated under these reaction conditions. This means isolation of the carboxylic acid requires neutralization of the reaction mixture.
Base-mediated amide hydrolysis is an irreversible process due to deprotonation of the carboxylic acid and formation of a non-electrophilic carboxylate anion. Isolation of the acid requires neutralization of the reaction.
Amides are the least reactive of the carboxylic acid derivatives and require harsh conditions to hydrolyse. Living systems can cleave amide bonds by using catalysts called enzymes. Different enzymes use different interactions to lower the activation barrier and accelerate hydrolysis under milder conditions. One day I might do a summary on such reactions (unless you are one of my 123104 students, in which case I've already written a brief description and you'll find it in the study guide).
Conclusion
Nucleophilic acyl substitution is an important reaction in synthesis and biology. All the various carboxylic acid derivatives can be prepared using variations of this substitution mechanism. This summary has looked at the reactions of carboxylic acids, acyl chlorides, anhydrides, esters and amides. At first glance, it looks like there a large number of reactions but, by looking at the mechanism, it becomes clear that they are all variations of the same reaction. The key to the reaction is the reactivity of the carbonyl group. It is polarized, making it a good electrophile, but it will reform if possible. In the case of carboxylic acid derivatives, it is always possible for the carbonyl to reform and eject a leaving group. There are effectively three variations on nucleophilic acyl substitution depending on whether the nucleophile is an anion or is neutral, and whether the reaction is acid-catalysed. Each of these reactions involves a nucleophilic addition followed by loss of a leaving group (also known as an elimination). Depending on the conditions and the functional group, there may be one or more proton transfers. Where these transfers occur in the reaction can change.
Carboxylic acids can be converted into all of the carboxylic acid derivatives (hence the name). The most reactive of derivatives are the acyl chlorides (or halides), followed by the anhydrides, the esters, and finally, the amides. The reactivity of these derivatives can be explained by looking at both the electronegativity of the leaving group on the carbonyl group and the possibility of electron delocalization. The reactivity can also be viewed from the perspective of how good a leaving group is attached to the carbonyl group. The better the leaving group the more reactive the carboxylic acid derivative. Good leaving groups are weak bases, or compounds with a strong conjugate acid (pK_aH is low).
Carboxylic acid derivatives at the top of the reactivity table are readily converted into those lower down the table. It is hard to convert one of the derivatives at the bottom up to a more reactive species. Remember, reactions generally occur to give the more stable compound. The classic table of carboxylic acid reactivity is given below:
The interconversion of carboxylic acid derivatives.