Condensation reactions of aldehydes & ketones: substituting the carbonyl oxygen atom
Introduction
Nucleophilic addition to an aldehyde or ketone occurs through a two step reaction mechanism. The reaction involves the nucleophilic attack on the polarized carbonyl to create a tetrahedral intermediate. There is then a proton transfer (or series of proton transfers) that delivers the product.
Hydrate formation by nucleophilic addition to an aldehyde. The mechanism is shown in the white box.
Certain nucleophilic additions can be accelerated by the addition of an acid catalyst. Hydrate formation is an example of this. The reaction has the same steps as nucleophilic addition but the order has been changed, there is a proton transfer first. This protonates the carbonyl group and makes it more electrophilic, which makes nucleophilic addition easier.
The mechanism of acid-catalysed hydration of an aldehyde. The order of the proton transfers has changed.
I have used reversible reaction arrows as the tetrahedral intermediate can either be deprotonated to give the hydrate or it can collapse back to the oxonium ion by eliminating water.
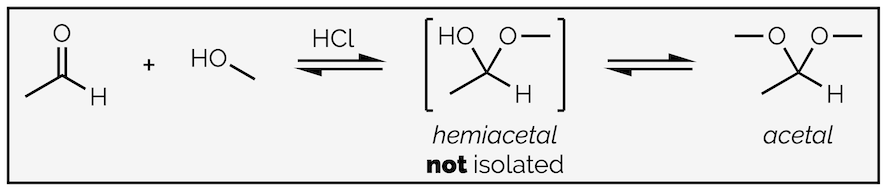
If an alcohol replaces water as the nucleophile then the mechanism above could give a hemiacetal but it could give a new product as well. The tetrahedral intermediate could eliminate the alcohol to return starting materials or it can lose water if the OH group is protonated. This gives a new oxonium ion and eventually leads to a new product to form. Now the product of the acid catalysed nucleophilic addition is a new functional group called an acetal. This is shown below:
The acid catalyzed addition of an alcohol to an aldehyde does not give the ‘expected’ hemiacetal but leads thee substitution of the oxygen atom and the formation of an acetal.
The initial nucleophilic addition occurs the same as before but the hemiacetal, the product of addition, cannot be isolated. It reacts further to substitute the oxygen atom of the carbonyl through the elimination of water in what is known as a condensation reaction. This summary discusses condensation reactions.
The fundamental mechanism in this summary is one of the longest most undergraduates will encounter. But, when broken down, it is just made up of repeating steps, and it isn’t as daunting as it first appears (I hope). Understanding this mechanism is really helpful as it lays the groundwork for so much that is to follow.
Substitution of the oxygen atom or the condensation reaction
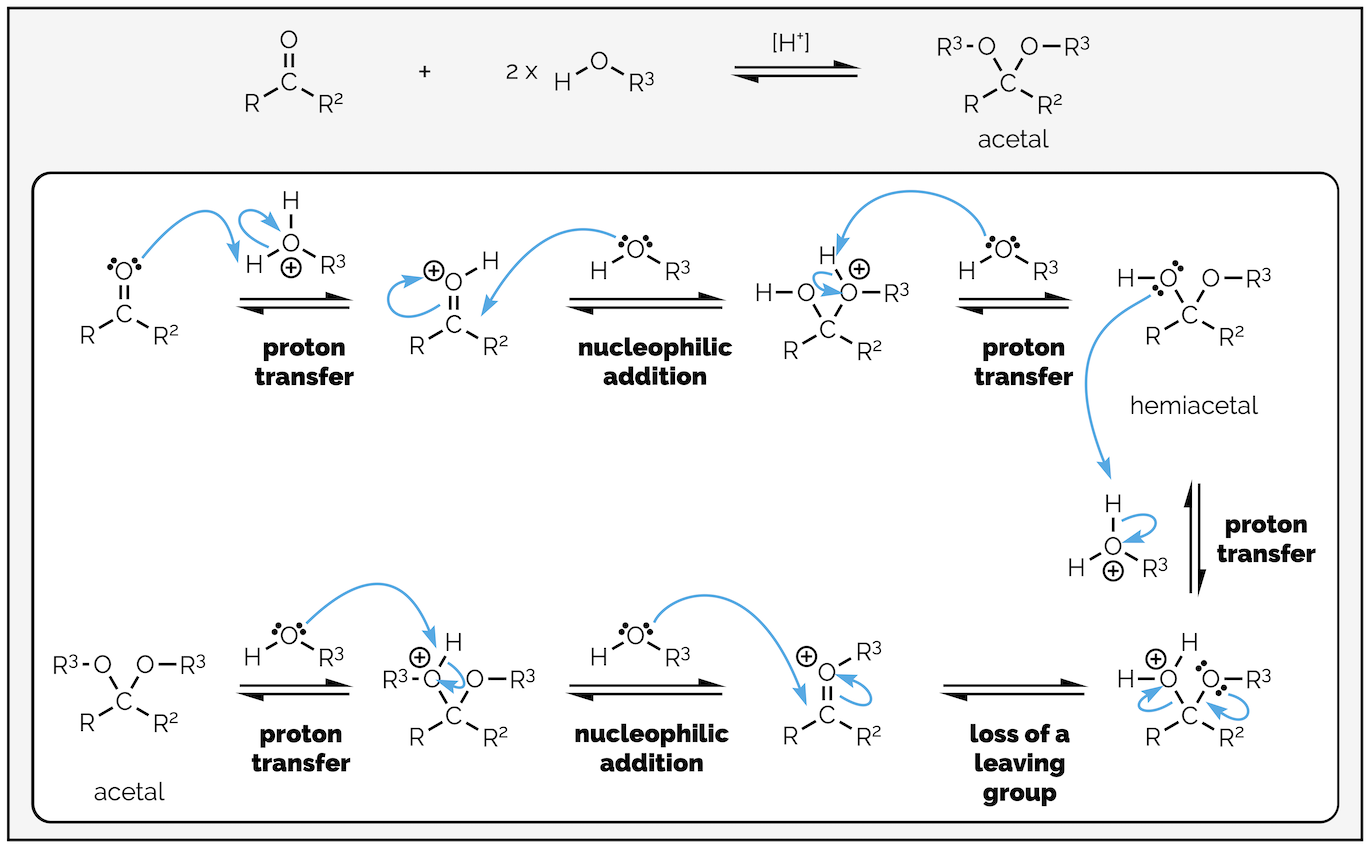
The general reaction equation for acetal formation is:
The general reaction for acetal formation has two equivalents of alcohol adding to the carbonyl group to substitute the oxygen atom and give an acetal and water.
The first part of the reaction mechanism is the same as acid-catalyzed nucleophilic attack leading to hydrate formation (or cyanohydrin formation, as shown in the previous summary HERE). In most organic textbooks, the acid is just written as a proton, H+. It is a useful simplification, as there are many species that could act as the proton source throughout the reaction, but it is also inaccurate and gets you into bad habits. A proton is just not stable in solution. As most of these reactions have an excess of alcohol the most probable proton source is a protonated alcohol (a kind of oxonium ion), and this is what I’ll show in my diagrams.
Hemiacetal formation. This involves two proton transfers and nucleophilic attack on the highly reactive oxonium ion. The original carbonyl oxygen has been highlighted.
After formation of the hemiacetal the mechanism can change. Either oxygen atom of the hemiacetal can be protonated. Protonation of the oxygen derived from the alcohol leads to the reverse reaction (from right to left above). If you don;t understand why formation of the carbonyl group is normally a favorable process (re)read the discussion of nucleophilic attack on the carbonyl group HERE. The favorability of the reverse process is why I have used equilibrium arrows.
Alternatively, the original oxygen, the one from the carbonyl group, can be protonated to form a new leaving group, and the reaction proceeds down a different pathway.
The second half of the acetal formation mechanism. The hemiacetal is protonated to create a leaving group. This is lost to give a highly activated oxonium ion, which is a good electrophile and is attacked by more alcohol.
Protonation of the oxygen gives an oxonium ion that looks like water connected to the tetraehdral intermediate, but note that it is charged and is known as a hydronoium ion. This is a good leaving group. A leaving group is an atom, or group of atoms, that takes two electrons away from a molecule. Good leaving groups are stable, relatively unreactive, species. They will be weak bases (or they will have a relatively strong conjugate acid; pKaH is low). Water is a good leaving group, being stable, and the pKa of its conjugate acid is low (the pKaH of water is 0).
The C=O double bond is strong, it is a double covalent bond and it has ionic character due to the polarization of the bond. It is readily formed, and a lone pair of electrons on the oxygen is shared between the two atoms as indicated by a lone pair to bond curly arrow. As there cannot be five bonds to the carbon atom the leaving group is eliminated, or kicked out as organic chemists like to say. This creates a new oxonium ion, a charged and hence highly electrophilic carbon–oxygen double bond. You should be able to see the resemblance between this and the oxonium formed by protonation. There is one big difference, the protonated species could lose the reactive positive charge by removal of the proton. Here there is no proton. Breaking a C–O single bond, the bond between O and R2 is hard, σ bonds are strong. Now the only way to remove the positive charge is to attack the polarized C=O bond and break the weak π bond. So another nucleophilic alcohol molecule attacks. This gives a protonated tetrahedral intermediate. Deprotonation leaves the acetal.
Comparing the two oxonium species. Both are activated carbonyl groups. One can be lose the charge by simple proton transfer, the other must undergo a reaction (nucleophilic addition) before it loses its charge.
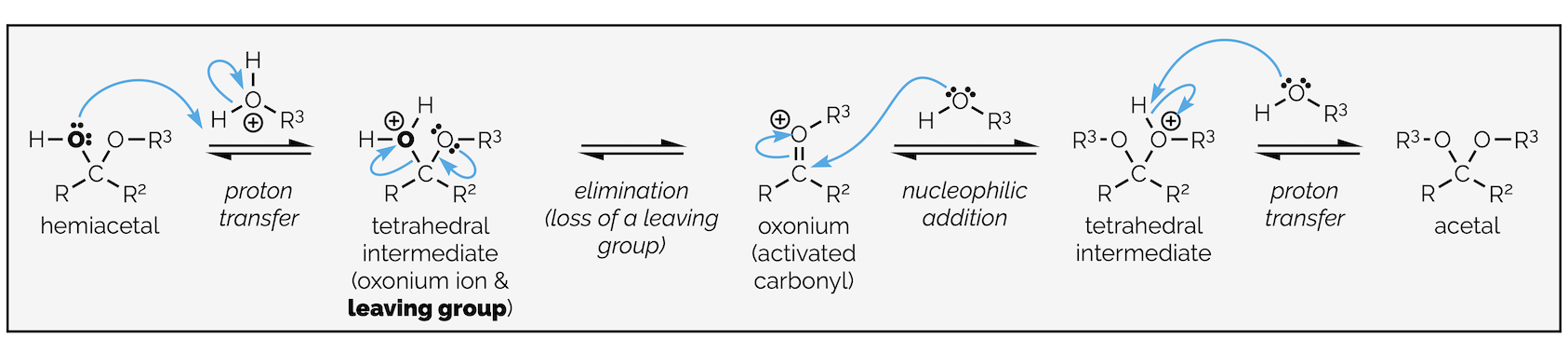
I told you it was a long mechanismm, and while it is long, it is not that difficult. It involves four proton transfers, two nucleophilic attacks and one loss of a leaving group. Effectively, the loss of the leaving group separates two repetitions of the standard nucleophilic attack on a carbonyl group. The whole mechanism is shown below:
The complete mechanism of acetal formation. It involves a proton transfer to activate the carbonyl group. This is followed by nucleophilic addition and a second proton transfer to form a neutral hemiacetal. A third proton transfer forms the leaving group, which is eliminated to give an activated oxonium ion. A second nucleophilic attack and a final proton transfer give the acetal.
The reaction is known as a condensation reaction because water is lost during the reaction. The reaction is not a substitution. The reaction involves addition and elimination only (loss of the leaving group). The following set of curly arrows is wrong. You must form the second oxonium species.
Addition of the second alcohol molecule is not a substitution reaction. There must be an elimination to form the oxonium ion first, then the alcohol adds to the cationic species.
Reversibility
Protonation of either oxygen atom of the hemiacetal or acetal leads to an oxonium ion and a good leaving group. Either water or an alcohol could be eliminated and both of these are weak bases. This means every step of the mechanism is reversible. The forward reaction is acetal formation, a condendation reaction, while the reverse is know as acetal hydrolysis, or the addition of water.
The problem for chemists is that the equilibrium favors the carbonyl group (see the discussion in the summary on nucleophilic addition HERE). To force the reaction to deliver acetal the equilibrium needs to be disrupted. Le Chatelier's principle tells you that if the equilibrium is perturbed the reaction will move in a direction that counters the change. In other words, if you increase the concentration of starting material, the reaction will shift to the right and form more product. More importantly in the context of acetal formation, if you remove water the reaction shifts forward giving more product (and more water). Another way of visualizing this is that by removing water from the reaction, you make one reaction step irreversible as you remove a nucleophile. This is shown in the diagram below:
Acetal formation is made irreversible by removing water from the reaction.
Water can be removed by using the correct solvent and glassware known as Dean-Stark apparatus. The water mixes with the solvent and forms an azeotrope that allows it to be removed by distillation. The Dean-Stark apparatus lets the solvent return to the reaction while allowing you to measure the amount of water removed.
Acetal hydrolysis is normally accomplished by adding an excess of water, which encourages addition of water to the oxonium species and leads to formation of the carbonyl group. The mechanism is the opposite of acetal formation.
Hydrolysis of an acetal to give the carbonyl compound, aldehyde or ketone, is normally achieved with an excess of water.
The reversibility of acetal formation is useful to organic chemists as it means acetals can be used as protecting groups for aldehydes and ketones. Protecting groups prevent functional groups from participating in unwanted reactions but can be removed to unmask the original functionality. The example below uses an acetal to prevent the Grignard reagent attacking the more reactive ketone and allows addition to occur to the less reactive ester. Once nucleophilic adddition is over, the ketone can be unveiled b y hydrolysis.
Acetal formation protects the ketone from nucleophilic attack (it no longer has a carbonyl group so is no longer electrophilic). The ester reacts and then acetal hydrolysis allows the ketone to be regenerated.
Examples of Acetal Formation (and analogous reactions)
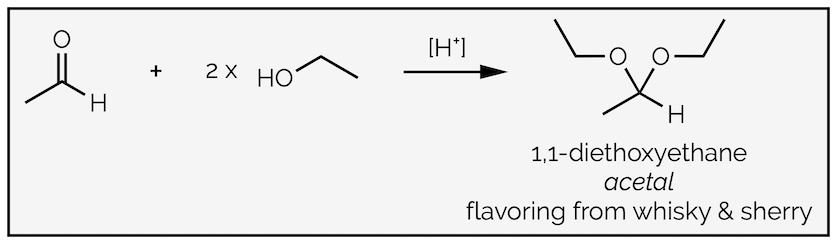
I hope I'm not the only organic chemist that has noticed this, but many students have no problem determining the product of the following reaction. This is application of the general reaction shown above with R2 replaced by something proper.
The formation of an acetal from an aldehyde and two equivalents of ethanol. This acetal is actually known simply as acetal and is a major flavoring in distilled drinks, such as whisky.
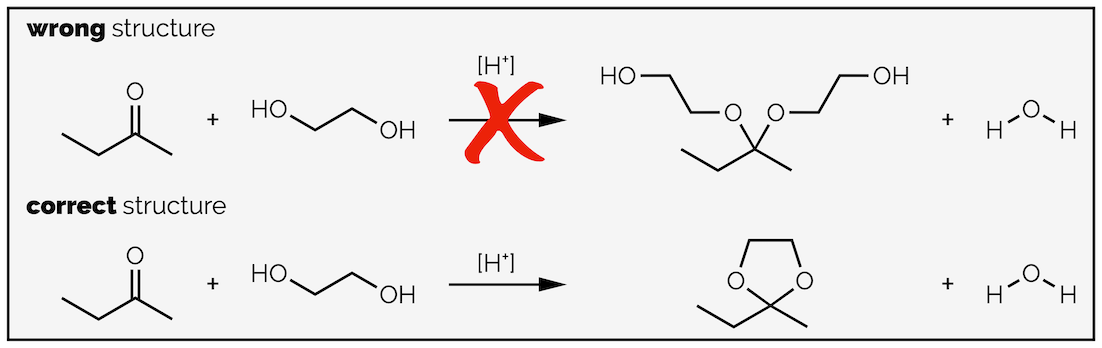
But the answers become more confused when the two hydroxyl groups are embedded in the same molecule. A frequent wrong answer is the acetal below formed by the addition of two molecules. The correct answer should have a cyclic acetal as the product. Cyclic (rings) acetals more readily formed and, once formed, are more stable than acyclic acetals. There are numerous explanations for these two observations. One explanation is that the oxonium species in the box rapidly undergoes ring closure due to the hydroxyl group being tied to it. The proximity of the alcohol also means it is more likely to attack than water which has to reach the molecule first. Also formation of the cyclic species is entropically favored; one ketone and one diol combine with the loss of water. There is no change in the number of molecules in the reaction or there is no change in disorder. If two molecules of alcohol add then there is a net loss in the number of molecules or a reduction in disorder and this is less favorable.
Diols normally give cyclic acetals.
The stability of cyclic acetals means they are more commonly used as protecting groups than their acyclic analogues. They are also found in numerous natural products and biologically interesting compounds. The agricultural fungicide, propiconazole, is an acetal:
Synthesis of the fungicide, propiconazole, which contains a cyclic acetal.
Cyclic acetals can also be formed if the alcohols and the aldehyde or ketone are in the same molecule. The synthesis of the spirocyclic olive fruit fly pheromone, olean, is shown below:
The synthesis of olean, another acetal.
Other nucleophiles will add to aldehydes and ketones to give acetal-like species. Sulfur is in the same group as oxygen so reacts in a similar manner (but with added smell). The most common thioacetals (thio- refers to sulfur and acetal because it still looks like an acetal) are the dithianes or six-membered cyclic thioacetals.
Thiols, the sulfur equivalent of an alcohol, behaves in the same manner and will form thioacetals by the same mechanism.
While I was happy to draw similarities between sulfur and oxygen to explain that the mechanism of thioacetal formation is the same as acetal formation, the presence of the sulfur means the reactivity changes. Thioacetals tend to be far more stable to hydrolysis than the corresponding oxygen molecules. They often need harsh conditions to convert back to the carbonyl compound. Additionally, the proton at the 2-position of a 1,3-dithiane (the proton of the aldehyde shown in the diagram above) is far more acidic than the proton of an acetal. Dithianes can be deprotonated and acts good nucleophiles. This property, combined with the ability to hydrolyse dithianes, allows you to convert the electrophilic carbon of an aldehyde into a nucleophile, a process known as umpolung. Finally, sulfur can be reductively removed from a molecule. This provides a route to reduce an aldehyde or ketone to a methylene group (CH2).
The chemistry of dithianes is different to oxygen-based acetals and allows for a variety of different reactions.
Nitrogen Nucleophiles
Amines participate in condensation reactions but the products look different to acetals. Nitrogen is in a different group and has a different valency so the change in product shouldn’t be unexpected. When nitrogen is neutral it has three bonds compared to oxygen’s two. The second difference is due to the increased basicity of amines compared to alcohols. Having highlighted the differences, it should be stressed that the reaction mechanism is going to be similar to above, and will comprise a series of proton transfers, one nucleophilic attack and one loss of a leaving group. If you have understood acetals you can understand nitrogen nucleophiles.
The overall condensation reaction for a primary amine with an aldehyde or ketone is shown below. The product is the nitrogen equivalent of an aldehyde or ketone, and is called an imine:
The reaction of an aldehyde or ketone with a primary amine results in the formation of an imine.
The first stage is nucleophilic addition. Unlike the previous examples, this occurs before protonation of the carbonyl group. The amine is more basic than the carbonyl and any acid in the reaction will protonate the nucleophile first. Of course, the protonated amine cannot react as it has no lone pair of electrons. Once nucleophilic addition has occurred the oxygen anion is the most basic species and this protonated. This gives a charged tetrahedral intermediate. A second proton transfer leads to the neutral species known as an hemiaminal. Funnily enough, considering its name, this is the nitrogen equivalent of the hemiacetal. It is also a tetrahedral intermediate and it will react further. These first two steps of the mechanism are reversed compared to acetal formation but otherwise this first stage is the same as above.
The first stage of imine formation involves formation of the hemiaminal. This is analogous to formation of the hemiacetal. The only difference is that order of the proton transfers has been changed. Here nucleophilic attack occurs before proton transfer.
The next stage is elimination of the leaving group, or, as organic chemists would say, kicking out water. These steps are identical to those in acetal formation. Yes, the nitrogen is more basic than the oxygen atom, but protonation of the amine just takes you back to starting materials as shown in the diagram above. Protonation of the oxygen atom allows the reaction to proceed.
Iminium formation. This is identical to formation of the oxonium ion during acetal formation. The oxygen is protonated and then eliminated to give the charged double bond species.
It is at this stage that acetal formation and imine formation diverge. The two charged species, the oxonium ion and the iminium ion behave differently. The oxonium ion is charged but it is not protonated. The charge makes it very electrophilic, and the only way it can loss the charge from the oxygen is if a nucleophile attacks. This can be either water (if the reaction is going backwards) or it can be an alcohol if the reaction is proceeding to the acetal.
Nucleophilic addition to an oxonium ion versus deprotonation of an iminium ion.
The iminium species is also charged but it is protonated. The iminium ion can lose the charge by deprotonated. Either the amine or water is sufficiently basic to do this. Proton transfer is a very rapid reaction, much faster than nucleophilic addition. So deprotonation leads to the neutral imine and there is no need for addition of another equivalent of amine.
Putting all of this together, the condensation of a primary amine and an aldehyde or ketone to given an imine is given below. This reaction is also known as Schiff base formation, with a Schiff base referring to a host of compounds containing a C=N bond.
The complete reaction mechanism for the formation of an imine (Schiff base reaction) from an aldehyde or ketone and a primary amine (or a whole host of other nitrogen-containing compounds).
Like acetal formation, every step of imine formation is reversible. As the carbonyl group is strong, the reverse process, hydrolysis of the imine, is normally the favorable reaction. The key to formation of the imine is removing water in the blue step above.
It is not just primary amines that react in this condensation reaction. Other nitrogen-containing compounds will give similar compounds by the same reaction mechanism. The two most common are oximes and hydrazones.
Formation of imine-like compounds. Oximes are made from hydroxylamine and hydrazones are made from hydrazine.
You may have noticed that throughout this discussion I have kept saying imines are formed from primary amines, what happens if you react an aldehyde or ketone with a secondary amine? The answer is that you get a different product. The iminium species formed from a secondary is not protonated. In many respects it is closer to an oxonium ion yet it reacts in a different way. The reason for this is that imines are less electrophilic. The C=N bond is less polarized than C=O bond as nitrogen is less electronegative. Additionally, there is amine present that can deprotonate the iminium species, just at the α position instead of on the nitrogen. This is leads to a species called an enamine.
The reaction of an aldehyde or ketone with a secondary amine results in the formation of an enamine by the mechanism in this diagram.
The first steps of the mechanism are identical to imine formation. Nucleophilic attack is followed by a series of proton transfers and loss of water as a leaving group. This gives the charged iminium species. The mechanism diverges at this point as there is no proton to remove from the nitrogen. Instead, the base removes a proton from the α-position. This creates a C=C bond and breaks the C=N bond giving the electrons to the more electronegative nitrogen and removing the positive charge from the species.
Enamines are an important species. They are nucleophilic on the α carbon. You should be able to draw a resonance structure that shows this carbon is electron rich. Using enamines as nucleophiles is important in nature, with many enzymes using similar chemistry to create C–C bonds. The basis of the 2021 Nobel prize in chemistry is using amines as organocatalysts with these reactions proceeding by the formation of iminium and/or enamines.
Conclusion
Nucleophilic attack on an aldehyde or ketone can occur with loss of the original carbonyl oxygen. Such reactions are known as condensation reactions. The mechanism for all these reactions are related, with the key step being formation of a good leaving group by protonating the hydroxyl group of the tetrahedral intermediate. The reaction is reversible. To drive it forward, so that the carbonyl compound is consumed, water must be removed. Hydrolysis, the addition of water to the compound, transforms the products back into the original aldehyde or ketone. When the nucleophile is an alcohol the reaction proceeds through a hemiacetal to give an acetal. These are important compounds found in nature and biologically active compounds. When the nucleophile is a primary amine the product is an imine, the nitrogen equivalent of the carbonyl group. If the nucleophile is a secondary amine, then the product will be an enamine. These are important compounds in the reactions of some enzymes and organocatalysts.
The concept of a leaving group on a tetrahedral intermediate is important. It was key for these condensation reactions and it will be important in the next reaction of the carbonyl group, substitution reactions of carboxylic acids and their derivatives.