Delocalization & Resonance
Introduction
Skeletal (line) drawings are a quick and easy representation of a molecule’s Lewis structure. While they are the most common method of communicating a molecule’s structure, they are far from perfect. Many molecules have more than one allowable, or correct, Lewis structure. Each of these drawings differs by the position of π bonds. The question is, “Which diagram is correct?”
Students hate the answer, which is “All of them”, that is why they are allowable! It turns out that Lewis structures aren’t very accurate. To improve the accuracy of line diagrams (basically, so that chemists can keep using them even though they are ‘wrong’), resonance and delocalization was introduced. This topic has confused undergraduates for as long as any of us can remember. Hopefully, this summary presents the basics needed to understand resonance and delocalization.
The Common Misconception
Before we get down to discussing resonance and delocalization, we need to address the source of most students’ issues with resonance and delocalization. Many molecules are best described by a series of Lewis structures. Each drawing fixes the electrons of a molecule in a single position. The allowable Lewis structure are linked together under the term resonance structures. Students start to think that the electrons are ‘moving’ between each set of discrete bonds or are resonating between each molecule. They start to think that each drawing is a different molecule. They are not. The problem is that line diagrams treat electrons as species that exist in a single position. Electrons aren’t, they’re not even particles moving in a molecule but are better thought of as a cloud that covers the entire molecule. Unfortunately, as soon as we say this, those students that haven’t wandered off to watch Netflix, picture a little ball rattling around a cloud that surrounds the molecule. This still isn’t right. The electron is the cloud. It is not in the cloud. The good news is we can ignore these clouds (that’s molecular orbital theory). The bad news is that we must remember that the resonance structures we’re about to draw are a useful model but not real.
Resonance Structures
What is an ‘allowable’ Lewis structure? It is a line diagram that does not break any of the rules of bonding. Ideally, all atoms (at least those of the second row of the periodic table) should have a full valence shell, or obey the octet rule. It is possible for atoms to have fewer electrons (often six electrons) but they will never have more (again, if they are in the second row). Atoms should have a formal charge if they are sharing their electrons in unusual ways (see the summary on formal charges for more information).
When can a molecule have multiple allowable Lewis structures? You can draw multiple line diagrams for any molecule that has conjugated π electrons. This means the molecule will have two, or more, π bonds separated by a single bond or it will have a 2p atomic orbital on an atom adjacent to a π bond. In such cases, the π electrons can delocalize or spread over the conjugated system.
If the π bonds or the 2p orbitals are separated by more than one σ bond then they are non-conjugated.
Examples of conjugation and non-conjugation
The different allowable skeletal representations, or resonance structures, for buta-1,3-diene are shown below. They are connected by a double headed arrow known as a resonance arrow. This indicates that the structures on either side are the same molecule. It is not a reaction arrow (➝) or the double equilibrium arrow (⇌). There are other resonance structures of buta-1,3-diene but they are less important (see later).
Resonance structures of buta-1,3-diene
The ‘real’ molecule is a combination of all of the resonance structures and is known as the resonance hybrid. In this structure the electrons are spread across the molecule. The double bonds have slightly more single bond character (they are longer) and the single bond has some double bond character (it is shorter than we would predict). This is represented with the dashed line indicating partial double bonds on all atoms.
A similar diagram shows the delocalization of a lone pair of electrons on an oxygen atom in a functional group known as an enol ether (en = alkene, ol = alcohol & ether = ROR). Note how in both diagrams the formal charges occur where there was a double bond. You cannot skip over atoms. This indicates that the electrons are still associate with the same group of atoms and are not moving to new atoms (this would be a chemical reaction).
Resonance structures of an enol ether (1-methoxyprop-1-ene)
Notice how the resonance hybrid indicates the polarization of the molecule. The δ+ shows that the oxygen has a slight or partial formal charge (not a whole +1 charge) and δ– shows the oxygen has a partial formal negative charge. This is important for reactivity and non-covalent interactions.
When drawing the possible resonance structures there are a number of guidelines you must follow:
No atom can exceed the octet rule (for the second row of the periodic table). An atom will have a full valence shell (8 electrons) or fewer but never more.
You can only move electrons not atoms
You can only move π bonds and lone pairs of electrons
The overall charge must remain constant. If a molecule is neutral, all resonance structures must be neutral (contain the same number of positive and negative formal charges).
Resonance structures and the curly arrow
Each resonance structure shows a different distribution of electrons within a molecule. Organic chemists depict the redistribution of electrons with curly arrows. Curly arrows indicate which electrons are going to be moved between drawings. One curly arrow represents the redistribution of two electrons with the tail of the curly arrow showing which electrons will change position and the head shows where the electrons will be drawn in the next resonance structure.
Curly arrows connecting allowable resonance structures
Curly arrows are helpful in quickly determining allowable resonance structures, and there will be a summary devoted exclusively to this topic. Curly arrows are more useful than just looking at resonance structures, they are also used to predict chemical reactions. We will be seeing a lot more of them in the future.
Resonance structures are all the same molecule
We cannot stress this too often: Each resonance structure is a different aspect or representation of the same molecule. The structures are not flipping between each representation. Imagine you take a picture of the palm of your hand and then one of the back of your hand. Both pictures show the same thing, your hand, and combined they give a much better idea of what your hand looks like. The pictures are resonance structures and your hand is the resonance hybrid. Another analogy comes from Marvel, and that is Spider-Man. We can describe the properties of Spider-Man by talking about a man (he has a head, two arms and two legs) and a spider (he sticks to walls). The man and the spider are the resonance structures allowing us to predict the resonance hybrid - Spider-Man, a man who can stick to walls and not a creature that is one minute a man and the next a spider.
In terms of chemistry, the resonance hybrid, the real molecule is a combination of all the resonance structures. It is almost* as if we were overlapping all the representations to give the real structure as shown below:
Representing resonance structures and the resonance hybrid
*We used the word ‘almost’ in the previous paragraph because resonance structures are not created equal. Some are more important than others.
Ranking resonance structures
It is possible to rank which resonance structures are more important, or contribute more to the resonance hybrid, than others. This becomes important if you are trying to rank the stability of anions to predict acidity or reactivity.
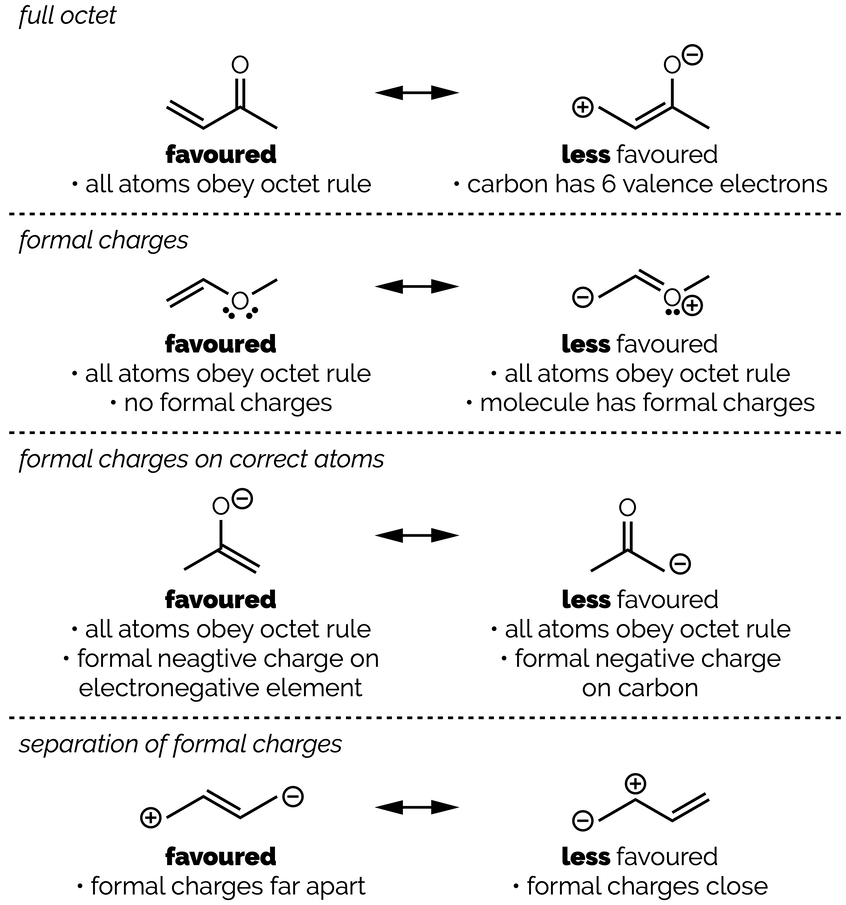
A resonance structure is more important, or makes a bigger contribution to the resonance hybrid, if:
All the atoms obey the octet rule (full valence shells are better than partially full)
There are no formal charges in the molecule
The formal charges are on the correct atoms (a negative charge is better on an oxygen atom than a carbon atom as oxygen is more electronegative)
Formal charges are far apart
Examples of the importance of various resonance structures
In organic chemistry courses, you will often encounter the saying ‘the more resonance structures a molecule has, the more stable it is’. This is not strictly true as the guidelines above outline - having favoured resonance structures is more important than having many resonance structures.
Delocalization, resonance structures and valence bond theory
Care must be taken when assigning hybridization to atoms involved in delocalization and resonance structures. It is easy to assign the incorrect hybridization. The key is to remember that all resonance structures represent the same molecule. The hybridization of an atom cannot change between resonance structures this would indicate that there were two different molecules. You must select a hybridization that fits all the resonance structures.
Incorrect assignment of hybridization in a delocalized system
As each atom of a delocalized system will be part of a π bond in at least one resonance structure it must have at least one non-hybridised 2p orbital. This orbital is required to form the π bond. In the diagram above, the resonance structure on the right has been drawn without a non-hybridised 2p orbital. It is incorrect. The correct assignment oh hybridisation is given below:
The correct assignment of hybridization in a delocalized system
There are two key points:
A lone-pair next to a π bond will be conjugated so must be treated differently when determining hybridization. This was discussed in the summary of hybrid orbitals.
Every resonance structure of a molecule will have the same collection of non-hybridized and hybrid atomic orbitals. This must be the case as every resonance structure is just a different representation of the same molecule.
Conclusion
The delocalization of electrons and the concept of resonance is really important for organic chemists. On one level it corrects a limitation of skeletal or line diagrams, allowing these simple molecular depictions to more accurately represent molecules. After that, it is found in nearly all aspects of organic chemistry from explaining physical properties like bond lengths and molecule shape, to being a tool to predict reactivity. If you cannot grasp resonance then organic chemistry becomes very challenging.
Practice what we’ve gone through with the worksheet HERE.