Reaction Mechanisms & the Curly Arrow
Introduction
Different chemists are interested in different aspects of a molecule, from electronic properties, catalytic activity to biological interactions, but at the heart of all chemistry are the molecules themselves. These molecules have to be made, and that requires chemical reactions.
As an organic chemist, I’m interested in the reactions that construct new carbon-based molecules with useful properties. I understand that there are different ways of thinking about or quantifying reactions. Some chemists might consider the molecule orbitals involved, some will use collision theory or transition state models while others are interested in the position of an equilibrium caused by the difference in free energy of the reactants. Some of these approaches will be the focus of other summaries but today I'm looking at the organic chemists use of Lewis structures and the curly arrows to describe reactions.
An example of how an organic chemist might represent the addition of cyanide to acetone to give a cyanohydrin.
The diagram above shows an example of what we consider to the 'sweet spot' between accuracy and simplicity. It shows the movement of electrons required to join two molecules, and provides a mechanism for the reaction. By breaking reactions down into the component parts, the mechanism gives you a framework to understand why electrons change position. The mechanism shows that most reactions follow a few simple principles, and that it is possible to use these principles to predict reactions rather than memorizing every reaction you meet.
To understand this approach to reactions, you will need to understand the structure of molecules (summary HERE) and the stability of various intermediates and/or species (see predicting acids/bases HERE). If you are uncomfortable with these topics start by re-reading the summaries (or an introductory chemistry textbook - a good free resource is LibreText).
Ionic versus Radical Reactions
There are two classes of reaction, ionic (or polar) and radical reactions. In ionic reactions, pairs of electrons are redistributed to make and break bonds in what is known as as a heterolytic process. In radical reactions, bonds are made and broken through the movement of single electrons or homolytic process.
Ionic (or polar) reactions versus radical reactions. Ionic reactions involve heterolytic bond formation and breaking through the movement of a pair of electrons while radical reactions involve homolytic bond formation and cleavage with one electron coming from each atom.
Below is an example of an ionic or polar reaction. While the starting materials are not charged like the cartoon in the previous diagram, the electrons are depicted as moving as a pair, the hallmark of an ionic reaction (that and the product is an ionic salt). The curly arrows drawn in blue show the movement of electrons in the reaction. They start with the lone pair of electrons on the nitrogen and flow in one direction to give a new bond and a new lone pair of electrons on the iodine atom. I'll discuss these arrows in far more detail later in this summary.
An ionic reaction showing a nucleophilic lone pair of electrons attacking the electrophilic carbon of iodoethane.
A radical reaction involves the movement of single electrons. These are represented on a molecule by a single dot (like a Lewis dot diagram). The example below shows the addition of a bromine radical (or atom) to an alkene. I have colored the atoms so that the alkene is the nucleophile and the bromine is the electrophile. Depending on your definition of a nucleophile and electrophile (see below), I shouldn’t have done this but (a) I think it is actually useful to think about the polarity of radicals, and (b) we’re not going to talk about radical reactions in this summary so just ignore that I any of wrote this.
All you need to know at the moment is that there are ionic and radical reactions.
The radical addition of bromine to an alkene to give a new carbon-centered radical.
Types of Ionic Reaction
When you first start studying organic reactions, it is useful to classify reactions as this makes communication easier but I want to stress that memorization is not important. When we start looking at the movement of electrons, it is the principles that determine where reactions occur that are more useful.
I’m going to start with four simple types of reaction (yes, this means there are other types of reaction that I don’t want to introduce at this stage):
1. Addition reactions
In addition reactions all the atoms of the starting materials are incorporated into the product. This is shown by the cartoon and example below:
Cartoon of an addition reaction followed by an example of an addition reaction, the bromination of an alkene.
2. Elimination reactions
These are the opposite of addition reactions and involve a molecule splitting apart.
An elimination reaction involves a reactant splitting into multiple molecules. It is is the opposite of an addition reaction. Here hydrobromic acid is eliminated from an alkyl bromide to give an alkene.
3. Substitution reaction
In a substitution reaction, two reactants exchange groups to form two new products:
A substitution reaction involves reactants swapping groups to make new products.
4. Rearrangement reactions
The starting material and products are isomers of one another. All the atoms of the product are in the starting material and it is just the bonding that has changed. Normally, this class of reaction is not important at first year but occasionally is mentioned in the context of tautomerization or the rearrangement of carbocations depending on the contents of the course/programme.
A rearrangement reaction. This is an example of an oxy-Cope rearrangement and there should be an additional rearrangement (tautomerization) to give an aldehyde not a enol.
These classifications aren’t silos that must be strictly adhered to. They help you think about reactions when you first starting studying at reaction mechanisms. Hopefully, they make it easier to see connections or similarities. Certain reactions can fit into more than one class depending on how they are drawn. Protonation of alkene is a good example (or does this mean bad example as it fits in more than one class?). Many textbooks would draw the reaction as below:
Protonation of an alkene as an addition reaction.
The alkene acts as a nucleophile and donates two electrons to attack the proton. This creates a bond between the carbon and the proton at the expense of the double bond. It gives a stabilized carbocation. As all the atoms of both starting materials have been incorporated into the product it is an addition reaction.
There is a second way of drawing the same reaction. It is arguably more accurate as, in solution at least, protons are too reactive to exist as shown (see the summary on acidity) and will be associated with a basic species. The drawing below shows the same reaction conducted with aqueous acid. Now all the molecules of the starting materials are not found in the product. This reaction is best described as a substitution as the proton has been exchanged between the reactants.
Protonation of an alkene shown as a substitution reaction.
Ultimately, it doesn't matter which of these mechanisms is used. The same principles apply, with an electron rich alkene acting as an electron donor (nucleophile; see below) and the proton acting as the electron acceptor (electrophile). It is the flow of electrons, and the reason they flow in a certain direction that is important, not the name of any process.
Types of Reactants
Reactions are caused by the attraction of charges or partial charges followed by a redistribution of electrons to make or break covalent bonds. Chemists divide reactants into two categories depending on their charge or polarization (although one class of reagent is further subdivided). Electron rich or negatively charged nucleophiles attack electron poor or positively charged electrophiles:
Nucleophiles
Nucleophiles donate two electrons to create a new bond (they are Lewis bases).
Nucleophiles will be electron rich species that will have either a lone pair of electrons or a high energy, reactive and polarized bond. A reaction is the redistribution of electrons and can always be thought to start from a source of electrons. This means reactions always start with a nucleophile attacking something. Examples of nucleophiles are:
Negative charge
Reactants with a negative charge will be nucleophiles. Most will have a lone pair of electrons that they can donate, although some, like the borohydride anion BH4 donate electrons from a bond.
Anions are nucleophiles.
Lone pair of electrons
Lone pairs of electrons on heteroatoms such as nitrogen, oxygen, phosphorus, and sulfur are good nucleophiles. Lone pairs of electrons can be considered non-stabilized as they are not shared between atoms. This means they are reactive. If the lone pair is shared by delocalization it will be less nucleophilic (amines are nucleophilic, amides are rarely nucleophilic).
A lone pair of electrons acting as a nucleophile.
Pi (π) bonds
Alkenes (& alkynes) and the π bonds of aromatic rings can behave as nucleophiles. Such bonds are relatively high in energy and hence reactive.
Alkenes, alkynes and aromatic rings have nucleophilic π bonds.
Polarized single (σ) bonds
Polarized bonds can act as nucleophiles. The electrons are closer to the electronegative atom (δ-) and this atom can be the nucleophile. Generally, this means that carbon will be the nucleophile when it is bonded to an electropositive atom (most atoms to the left of it on the periodic table or metals).
Polarized sigma (σ) bonds can be nucleophiles. The more electronegative atom will be the nucleophilic atom (and the reaction above is not a good example as there are a number of side reactions that can occur but that is a story for another day).
In many undergraduate courses, organometallic reagents like the one above are considered as anions instead of having a carbon–metal bond. A molecule like ethyllithium (above) is often considered to be an ethyl anion (Et- or CH3CH2-).
Nucleophiles can be further subdivided to give a special class of nucleophile called a base. Bases donate two electrons to create a new bond to a proton. Nucleophiles can be said to donate two electrons to create a new bond to everything else.
Nucleophiles and bases are subtly different mostly as a result of the difference in size between a proton and all the other atoms. This leads to the rough rule of thumb that states that nucleophiles can behave as bases but not all bases will behave as a nucleophile. Hydride, H– or a hydrogen anion, is a good base but never a good nucleophile. Below is an example of the hydroxide anion behaving as either a base or a nucleophile depending on what atom it attacks.
The same reagent, in this case a hydroxide anion, can be both a nucleophile and a base depending on where it reacts in a molecule.
The top row (above) shows a substitution reaction with the hydroxide anion attacking a carbon atom and forming a new C–O bond. In this example, the hydroxide is donating two electrons to create a bond to carbon so it is a nucleophile. Reacting the same reagents can also give an alkene by elimination. In this reaction (bottom), the hydroxide anion has attacked a hydrogen atom or caused deprotonation. It is donating electrons to a proton so has acted as a base. More advanced organic chemistry courses will tell you how to predict which reaction is favored.
The Strength of a Nucleophile and/or Base
One of the statements above leads to a common misconception, and that is that the 'strength of a nucleophile' (nucleophilicity) and the 'strength of a base' (basicity) can be used interchangeably. They cannot. The two concepts are very different even though the reactants are often the same. Nucleophilicity is a kinetic property. A stronger nucleophile attacks an electrophile faster than a weak nucleophile. The strength of a base (basicity) is a thermodynamic property. The stronger a base the further the equilibrium lies to one side (normally the right). This is a measure of the energy difference between the protonated (acid or conjugate acid) and deprotonated (base or conjugate base) forms of the compound. The rate of reaction has nothing to do with it.
This means a strong nucleophile can be a weak base and vice-versa. Often the two properties get linked (and you'll see an example below). There are similarities but there are also important differences. Just remember that nucleophiles and bases do different things, they react with different parts of a molecule and are different.
The relative strength of two nucleophiles depends on how readily they can donate two electrons. This is related to electronegativity. Here are some guidelines:
1. Anions are more nucleophilic than neutral species
Anions are more electron rich than neutral species. This means they are normally good electron donors. But you have to be comparing the same atoms. So perhaps it is better to write that the conjugate base (A–) is always a better nucleophile than the acid (HA).
2. Nucleophilicity decreases as you move across a row of the periodic table.
As you move from left to right across the periodic table so electronegativity increases. If an atom holds onto its electrons more tightly it will be a poorer donor. Across a row, the nucleophilicity will often match the pKa value, although nucleophilicity is more strongly affected by sterics while acidity/basicity isn't.
3. Nucleophilicity increases as you go down a group of the periodic table.
This is the opposite of basicity, and it relates to the polarizability or electronegativity of the atoms. The bigger the atom, the more polarizable it is. The electrons are held more loosely and can shift more readily. They are influenced by their surrounds more and easily shared. Simply, the atom is less electronegative.
4. Other factors.
Nucleophilicity is influenced by many factors. Other than those listed above, the two most important are solvent and steric bulk (size). Neither are generally discussed in introductory chemistry courses. Solvation, the interaction between solvent and substrate, can reduce nucleophilicity if the solvent forms a barrier around the reactant. This means polar protic solvents reverse the trend in guideline 3. Steric hindrance behaves in a similar way. The bulkier the molecule, the less nucleophilic as you are effectively adding a shield or barrier to the reactant.
Electrophiles
Electrophiles accept two electrons to create a new bond (they are Lewis acids).
Electrophiles are electron poor species. The electrophilic site will either be a positively or partially positively charged atom. This means electrophiles either don't obey the octet rule or contain electronegative atoms.
In terms of orbitals involved, electrophiles will have a low energy empty orbital (LUMO). This can either be a atomic orbital in positively charged species or molecules containing group 13 elements, or it will be a low lying antibonding orbital associated with a bond to an electronegative element.
Nucleophiles attack electrophiles and a curly arrow will always point towards the electrophile. Examples of electrophiles are:
Positively charged species
The simplest electrophile is H+, the hydrogen cation or proton. It has no electrons and an empty 1s atomic orbital.
Protons are good electrophiles (although arguably it is the oxonium adduct that is the real electrophile and this should be listed in a different category … don’t you love chemistry?)
Carbocations have only six valence electrons and have an empty 2p atomic orbital. They are good electrophiles as they want to gain two electrons and fill the valence shell.
Carbocations only have six valence electrons and an empty 2p atomic orbital, they are good electrophiles.
Neutral Electrophiles
Neutral compounds containing a group 13 element are electrophilic as the group 13 element will have only six valence electrons. Reacting as an electrophile by accepting electrons allows the atom to gain a full octet. In terms of orbitals, such species have an empty p atomic orbital that is readily attacked.
Group 13 elements form neutral species while still having an atom with only six valence electrons. They are electrophilic. Add electron withdrawing or electronegative elements like fluorine and you will increase the electrophilicity.
Molecules with polarized bonds can be electrophiles. Electrons are pulled towards the most electronegative atom making this atom partially negatively charged (δ-). The other end of the bond is partially positively charged (δ+) and is the electrophilic site. The carbonyl group is an important example of a polar bond with an electrophilic carbon atom.
The electronegative oxygen atom polarizes the carbonyl group leaving the carbon atom partially positively charged. The δ+ carbon is an electrophilic site.
Polarized single (σ) bonds can give an electrophilic carbon atom if the other end of the bond is an electronegative atom. The classic example of this is with alkyl halides.
Bonds to electronegative atoms leave the carbon atom partially positively charged (δ+) and make it an electrophilic site.
For those that want a little more detail (very little), the electrophilic site in a molecule will be a low energy empty orbital, the lowest unoccupied molecular orbital (LUMO). In the case of a proton this is the empty 1s atomic orbital. With carbocations and group 13 elements it is an empty 2p atomic orbital. For the polarized bonds, it is slightly more complicated being an antibonding orbital, either σ* or π*. The more electronegative the atom, the lower in energy its orbitals are and the lower in energy or more reactive the antibonding orbital will be. It is possible to understand reactions without recourse to thinking about the orbitals, and, if I'm honest, I think most students taking first year chemistry don't need to go into this kind of detail.
The Strength of an Electrophile
It should come as no surprise that positively charged electrophiles are more reactive than neutral species.
The charged species is more electrophilic than the neutral species.
The electrophilicity or strength of the electrophile will be influenced by substituents attached to the electrophilic atom. Alkyl groups, which are electron donating through the inductive effect (or hyperconjugation), reduce the electrophilicity. This can be seen in the reactivity or stability of carbocations. More substituted the carbocation the more stable it is and less electrophilic.
Electron donating groups, such as alkyl groups, can reduce the electrophilicity of an electron poor site.
Delocalization of a carbocation also reduces the electrophilicity by stabilizing the charge over a greater number of atoms. Compare a secondary carbocation to the reactivity of an allyl (or crotyl) cation:
The delocalization of electrons will influence electrophilicity. The allyl cation is less electrophilic than a simple secondary carbocation.
Similar effects will influence the electrophilicity of neutral species. The electrophilic strength of an atom depends on the extent (size) of the partial positive charge (δ+). Adding electronegative atoms as substituents increases the partial positive charge and makes the site more electrophilic. An acyl chloride is more electrophilic than a ketone as the chlorine atom drags electrons away from the carbonyl carbon.
Both inductive effects and the delocalization of electrons can alter the electrophilicity of a reactive site.
Conversely, electron donating groups, those that allow delocalization of electron density into a carbonyl group can reduce the electrophilicity. An amide is less electrophilic than a ketone due to the delocalization of the nitrogen lone pair. The delocalization pushes electrons onto the carbonyl group and makes the carbon less partially positive (with nitrogen delocalization or the mesomeric effect is more important than electronegativity).
Summary Of Nucleophiles and Electrophiles
A summary of nucleophiles and electrophiles.
Reaction Mechanisms and the Curly Arrow
In many of the examples above, I have used curly blue arrows to describe the movement of electrons in a reaction. This section gives a brief introduction to this powerful tool.
A reaction mechanism describes the order in which bonds are made and broken. By understanding reaction mechanisms you will see that reactions follow a limited number of core principles. By following electrons it is easier to understand chemistry rather than trying to memorize every reaction you encounter.
Curly arrows offer a useful way to think about reactions, to predict the structure of products but they are not real. Reaction curly arrows are used in the same way resonance curly arrows were (see previous summary HERE), they connect two sets of skeletal diagrams by showing which electrons in the starting materials (the left-hand side of a reaction) will move and then indicating where these electrons will be drawn in the diagram of the product (normally, the right-hand side of the equation). Real reactions don’t occur by bonds moving like this but just because curly arrows aren’t real you should never underestimate the power of this model. For all its inaccuracies, it is shockingly good at predict reaction outcomes.
Both resonance and reaction curly arrows show the movement of two electrons, but there is a fundamental difference between resonance and reaction curly arrows. The curly arrows connecting two resonance structures do not describe a real process, they simply help you to connect two, or more, pictures. A set of reaction curly arrows connect diagrams and describe real change. They describe bonds being made and broken.
The difference between resonance and reaction curly arrows.
A reaction curly arrow always means the movement of two electrons (radical reactions use curly fishhooks or half-arrows to indicate a single electrons is moving). The tail of the arrow indicates which pair of electrons is moving. The head of the arrow shows where the electrons move to. This is where they should be drawn in the next diagram. Unfortunately, you will meet numerous, subtly different, conventions for drawing curly arrows depending on the textbook or website. The most common are below:
Different conventions for drawing curly arrows.
I tend to use the second, but, annoyingly, I will use any of them if I think it helps communicate my point (a lie, I never use the bouncing curly arrow). The third, showing the position of the newly form(ing) bond as well as the curly arrows is probably the clearest to follow (but I'm lazy ... I’ll try and make an effort to draw it but it is so much quicker not to! Sorry).
Allowable curly arrows
When drawing a reaction mechanism you will find three allowable curly arrows:
1. Lone pair to bond
The tail of the curly arrow will start on an atom that has a lone pair of electrons. This can be a heteroatom or a carbanion. The head of the arrow will point to where you will draw a new bond in the next diagram. The new bond can either be a single (σ) or double bond (π; some might argue that lone pair to π bond is a resonance arrow but I would tell them to stop complicating things like the reactions of enolates).
2. Bond to lone pair
Breaking a bond. The tail of the curly arrow starts on the bond to be broken and the head goes to the atom that will gain a new lone pair of electrons. The atom gaining the lone pair of electrons must be one of the two forming the original bond.
3. Bond to bond
Breaking and making bonds simultaneously. The tail of the arrow starts on the bond that is breaking and the head of the arrow will go to a point between the two atoms being joined. One of the atoms must have been involved in forming the original bond. The most common examples of this arrow are between a double bond and another atom but reducing reagents like the borohydride anion show that a σ bond can be used to form a new σ bond. Bond to bond curly arrows show the most variety in terms of conventional curly arrow versus atom specific curly arrow versus bouncing curly arrows.
Conservation of charge
At undergraduate level, organic reaction mechanisms only involve the movement of electrons. No electrons are created or destroyed (arguably this only occurs when you start looking at electrochemistry). This means the overall charge of a reaction never changes. In the three examples above, the starting materials or left-hand side, have a positive charge. As you are only moving electrons, the product or right-hand side of the equation, must also have a positive charge. This is the same as you saw when discussing resonance structures and resonance curly arrows.
The overall charge must be the same on both sides of the reaction.
But, be aware, chemists can be lazy and will add a proton or water to remove negative charges from the reactants without ever worrying about showing the balance of charge. This will only occur after the rest of the reaction has finished.
Reaction mechanisms
When drawing a reaction mechanism, it is common to see the three allowable curly arrows grouped together in the following processes, mechanistic steps or reaction steps:
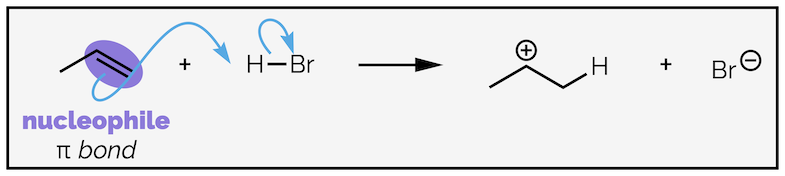
1. Nucleophilic attack - Most reactions involve a pair of electrons attacking an appropriate electrophile. Often nucleophilic attack is accompanied by a bond breaking.
- Proton transfer - one of the most important reactions (and a very specific example of nucleophilic attack). It should always involve two arrows, one for the proton transfer and one breaking the H–A bond, but chemists are often lazy (sorry, I keep typing this a lot, but it is true when it comes to their diagrams (not true when it comes to just about anything else)) and will use H+ and use a single arrow.
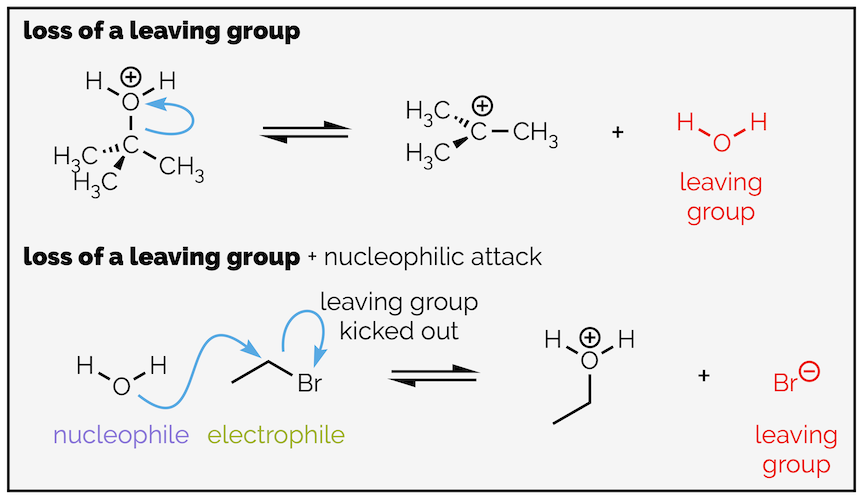
3. Loss of a leaving group - this is the breaking of a bond (and yes, it often accompanies nucleophilic attack). A leaving group takes electrons away from the reactant. A good leaving group will be relatively stable and unreactive. It will be a weak conjugate base or weak base.
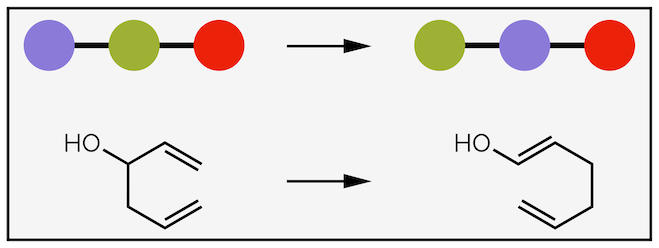
4. Rearrangements - the curly arrows shows the redistribution of bonds and atoms within a single molecule. You will not meet such reactions at the start of your organic chemistry career (so I don't have to discuss why I've drawn the curly arrow ‘upside down' (it is not an error) or why I haven’t drawn the delocalization).
Not all reactions involve the direction conversion of the starting materials into products through a single step or process. Some reactions require multiple reaction steps. The sequence of elementary reaction steps is known as the reaction mechanism. Each step is indicated by a straight (non-curly) reaction arrow, and leads to an intermediate that then undergoes a subsequent elementary step. The elementary steps correspond to the basic reactions described above (addition, elimination, substitution, proton transfer and rearrangement).
A reaction mechanism can involve multiple steps or ‘mini’ reactions that bring about the overall transformation.
If multiple reaction arrows separate the starting materials from the product then the reaction occurs by a stepwise mechanism. The number of curly arrows drawn before each reaction arrow is important. If two curly arrows are drawn on the same side of the reaction, they indicate that the bonds are being made and broken at the same time in the same step or in a concerted manner. If there is only one curly arrow, then only one bond is being made or broken in that step.
A word of warning: I have noticed a reluctance by students to draw molecules. Often they prefer to draw a molecule once and then add as many curly arrows to it as possible. (a) This tends to lead to confusion as to what bonds are being made and broken (or is a sign of confusion?); (b) it is frequently wrong. Surprisingly few reactions are completely concerted.
The number of steps is important. Don’t try to fit all curly arrows on a single diagram (unless the reaction is concerted e.g. epoxidation with a peracid). Hemiacetal formation is stepwise (although it could have been drawn one step shorter with an internal proton transfer but I was trying to make a point).
In a later summary, I will go through how you can determine the number of steps in a reaction.
Trying to Draw a Reaction Mechanism
Being able to write a reaction mechanism is a useful skill, but it is challenging. It takes a long time to master. For many students taking a single organic course, I'd argue it is unnecessary but the logic outlined below is useful. Thinking stepwise about how things happen is useful. Outlined below are some principles that help think about reaction mechanisms logically. They help you develop a structured approach to thinking about reactions.
- Draw the reactants and reagents in full.
- Add lone pairs of electrons and the polarization of bond.
- If you are given the structure of the intermediates, identify the bonds broken and formed. These signpost which atoms you are interested in.
- Identify the nucleophile - the electron rich molecule that uses electrons to attack another group.
- Identify the atom or bond that acts as the nucleophile. It will be part of the new bond.
- Identify the electrophile - first the molecule and then the atom.
- Draw a curly arrow starting from the nucleophile (atom or bond) and going to the electrophilic atom:
- The arrow must start from a lone pair or bond
- If the nucleophilic atom was neutral it will be positively charged in the product/intermediate
- If the nucleophilic atom was negatively charged it will become neutral in the product/intermediate
- If the new bond is formed to a positively charged electrophilic atom, the atom will become neutral
- If the new bond is formed to a neutral or uncharged electrophilic atom (H, C, N, or O) then there must be a second curly arrow breaking a bond and taking electrons away (loss of a leaving group)
- The second arrow should flow in the same direction and go towards a weak base (either electronegative atom or resonance stabilized system)
- The overall charge of the reaction remains the same and any negative charges should be stabilized.
Example
Draw the curly arrows for the following reaction mechanism for esterification of an alcohol using an acyl chloride.
To achieve this task follow the guidelines given above. You can ignore the first, as I have already drawn each molecule out in full but you should add the lone pairs of electrons and the polarization of the bonds. Just do this one step at a time.
This should help you identify the nucleophile and electrophile. The nucleophile is an electron rich area that will donate electrons to create a new bond. Any of the lone pairs of electrons fulfil this criterion. Which set is it? Well there are two ways of determining this. The simplest is by inspecting the intermediate. It has a new bond between the oxygen of the alcohol and the carbon of the acyl chloride. The only one of these atoms with a lone pair of electrons is the oxygen atom. It is the nucleophile. Alternatively, if you inspect each atom you can see that of the two oxygen atoms, the alcohol is the better donor as it is sp3 hybridized compared to the carbonyl, which is sp2. The sp3 orbital is more available for donation as it is larger (read the summary on acidity and basicity if you need an explanation of this). The chlorine atom is electronegative and attached to an electron withdrawing carbonyl group. It doesn’t donate its lone pairs.
The electrophile is the atom accepting two electrons to form a new bond. Again, you can identify this atom by looking at the product and see where the new bond is. The new bond is formed between the oxygen atom of the alcohol and the carbon of the carbonyl group. The oxygen is the nucleophile so the carbon must be the electrophile. It is also the most electron deficient atom due to being attached to an electronegative oxygen and electronegative chlorine atom.
The nucleophile attacks the electrophile. A curly arrow starts with the electrons of the nucleophile and it goes to make a bond to the carbon. I have drawn a dashed line to show where the new bond is formed, and the curly arrow goes to this (slightly closer to the carbon to avoid ambiguity). This is an example of nucleophilic attack.
The carbon atom is neutral so you must break a bond to take two electrons away (the carbon atom cannot have ten valence electrons, it cannot break the octet rule). The electrons could go to the oxygen or the chlorine atom. The questions shows that they must go to the oxygen. I’ll discuss why this is in a different summary (but the double bond or π bond is weaker than a σ bond and the oxygen is more electronegative).
Step 2 - This step occurs within a single molecule. Identifying nucleophile and electrophile is slightly less meaningful. I would start by identifying the bonds that are made and broken. A double C=O bond is formed and the C–Cl bond is broken. The oxygen starts negatively charged and becomes neutral. It has lost a share of electrons. The chlorine is the opposite. The negative charge on the oxygen suggests it is electron rich. The curly arrow will start here and go to the bond to create the double bond. The carbon cannot have ten valence electrons so there must be a leaving group that can take electrons away. This is the chlorine atom. The second curly arrow is an example of loss of a leaving group.
Step 3 finishes the reaction mechanism. Identify which bonds are made and broken. Identify the polarization of the bonds. Identify which atom acts as a nucleophile. It will be electron rich, either negatively charged or partially negatively charged. It will also be part of the newly formed bond as it will donate electrons to form the new bond. It will attack the electrophile, this will be electron poor. It will be either positively charged or partially positively charged. Take care here. The electrophile will be part of the new bond. The electrophile will be at the electron poor end of a bond. This means the positive charge tells you which molecule is the electrophile but not which atom. The formal positive charge is not on the electron poor atom of the bond. Formal charges are electron book keeping and not 'real’ (hence the formal bit). The polarization of the bond is determined by the electronegativity of the atoms.
With all this information you can now draw the final curly arrows. These make a proton transfer.
The mechanism is:
Conclusion
This summary introduced how organic chemists rationalize reactions through the use of the curly arrow. The order in which bonds are made and broken in a reaction is known as the reaction mechanism and the curly arrow can you used to describe the redistribution of electrons in each of the steps (parts of the reaction) of the mechanism. To be able to accurately draw the curly arrow reaction mechanism, you must be able to identify which bonds are made and broken. You must also be able to identify which atoms or bonds are used to create a new bond. These are electron donors or nucleophiles. Nucleophiles attack electron poor atoms, which act as electron acceptors and are called electrophiles. In subsequent summaries, I shall look at different reactions in more detail.