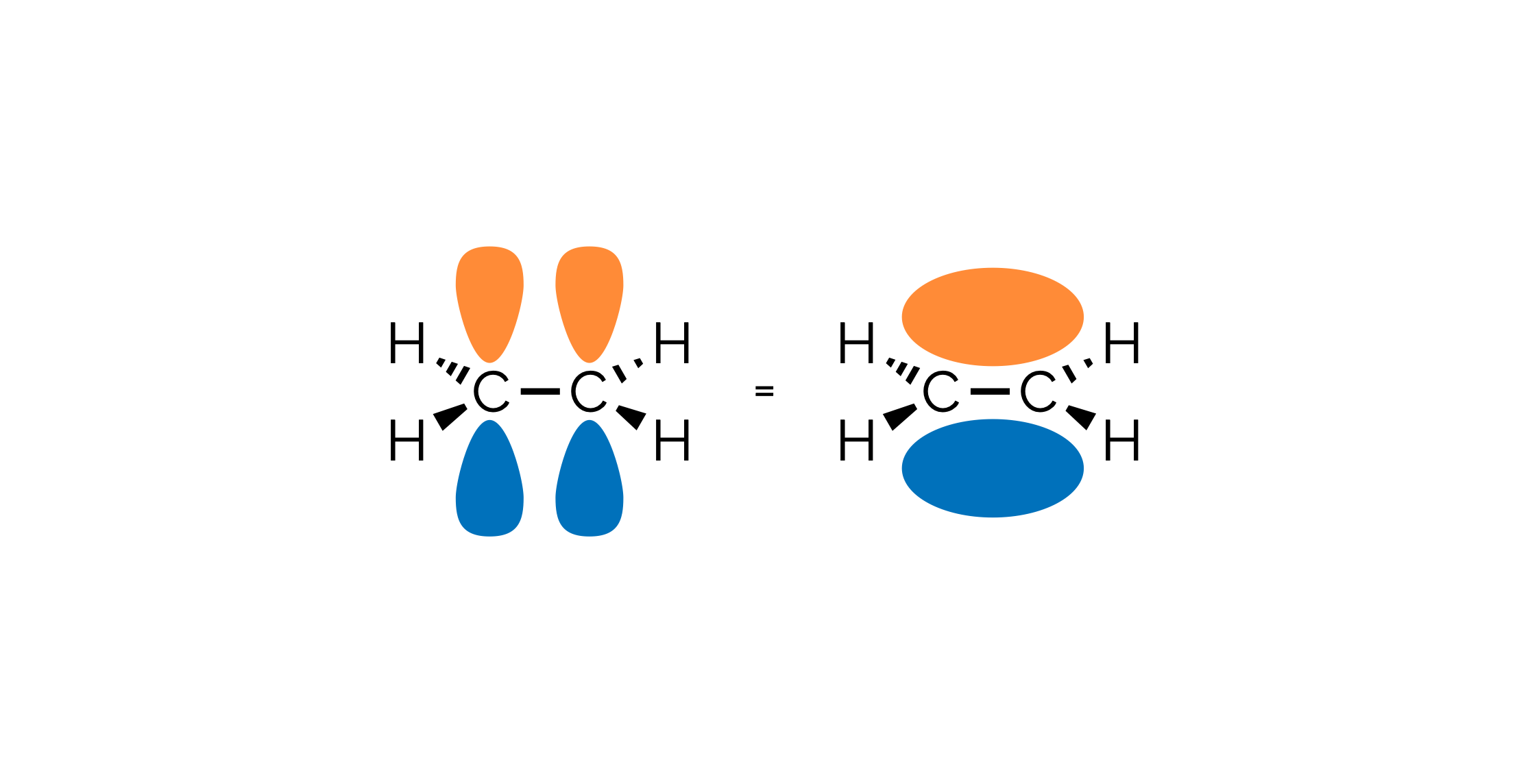Le Chatelier’s Principle: Stressing Equilibria
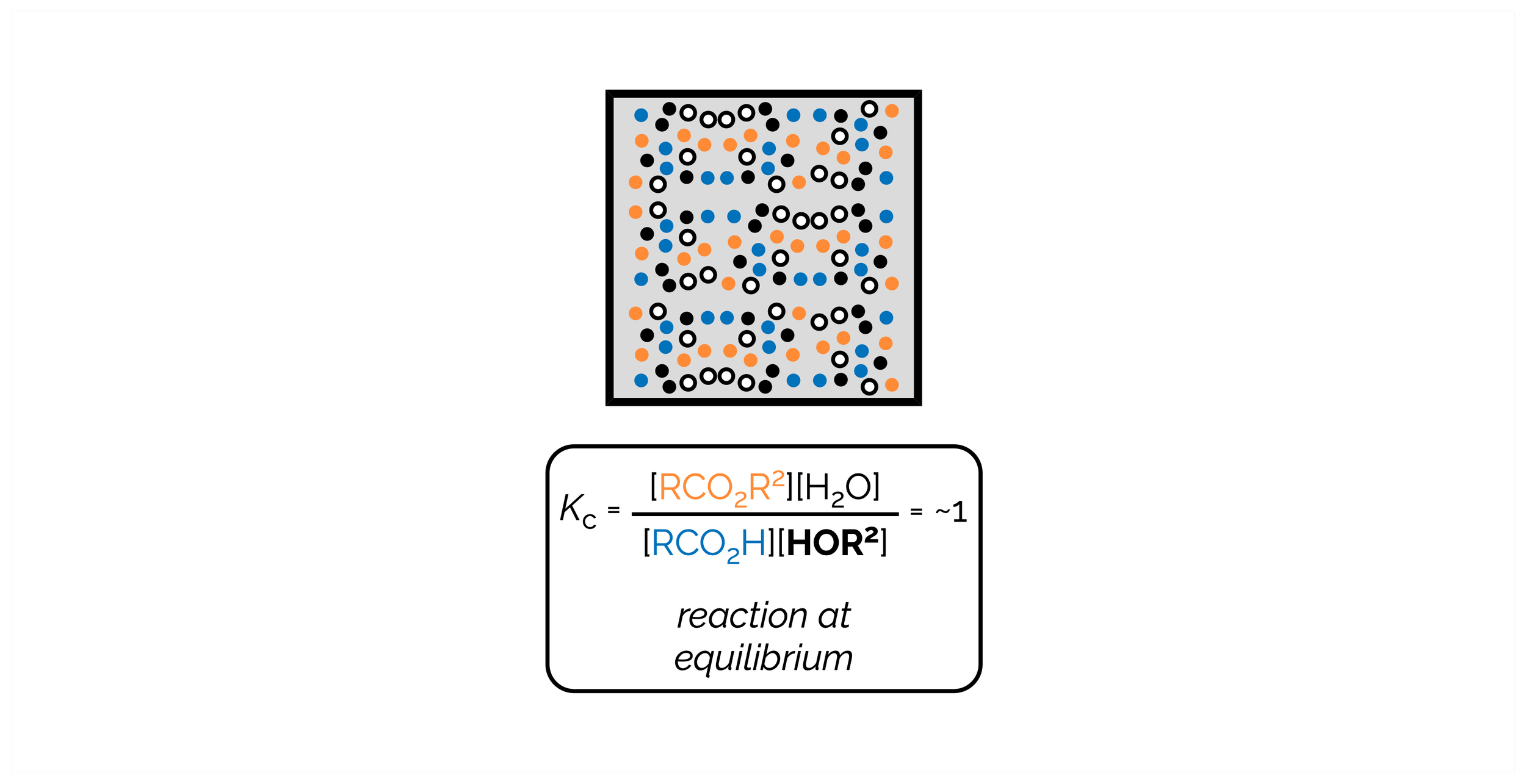
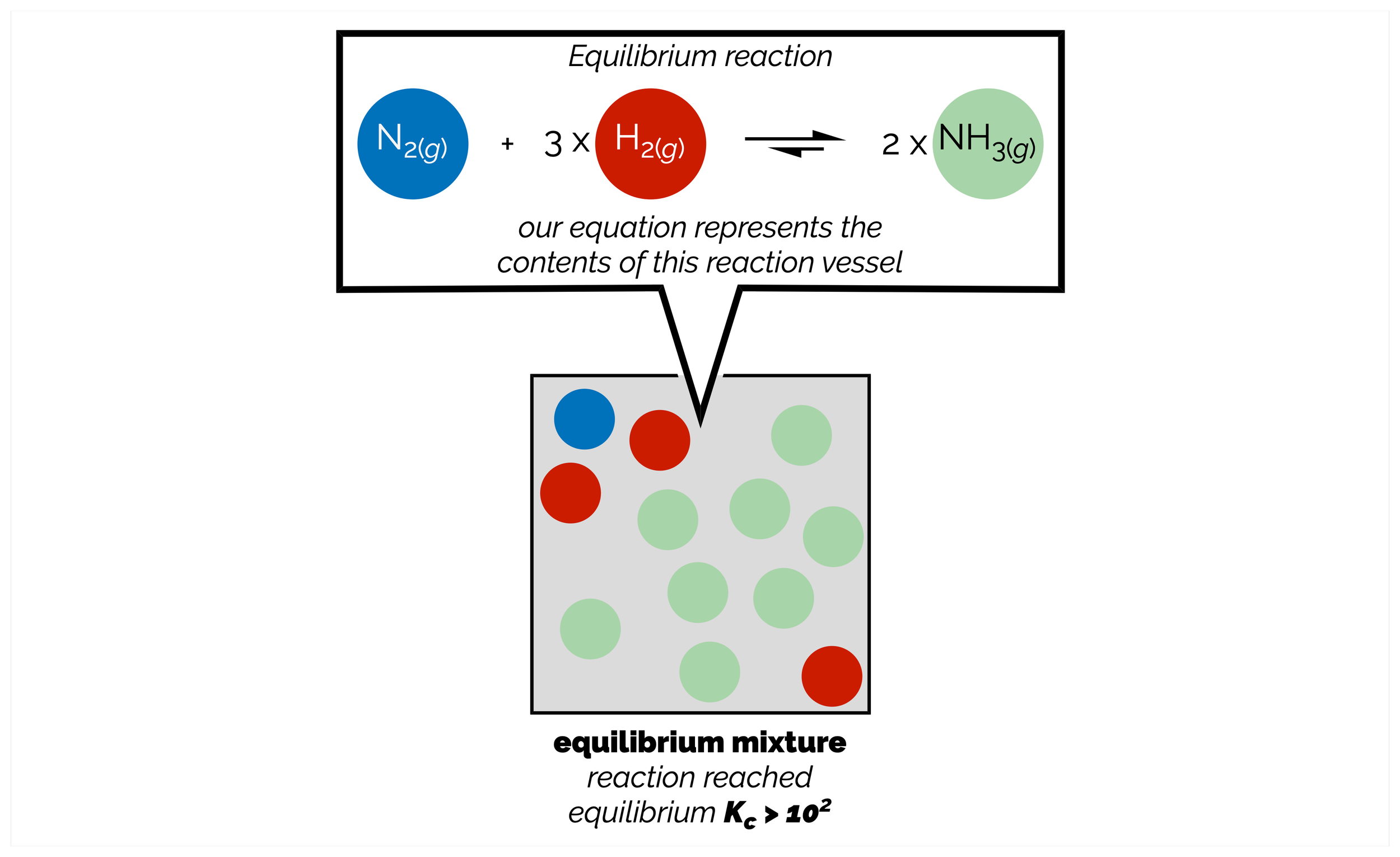
Reactions are equilibrium processes. The position of the equilibrium, how far the reaction goes either forward or backward, is given by the equilibrium constant. This is a constant as long as the conditions remain unchanged. When you alter any aspect of the reaction, or put a stress on the equilibrium, it will no longer be at equilibrium. The reaction will move in a direction that counters the stress. This means the reaction changes the concentrations of the reactants or products to return the system to an equilibrium. These changes can be described by Le Chatelier’s Principle. Chemists use this principle to push a reaction in a specific direction and form more of a desired compound.
Equilibria (by a synthetic chemist)
Many synthetic chemists, like myself, forget that reactions are a chemical equilibrium, that ultimately reach a position where the forward and reverse reactions occurring at the same rate. For most (successful) organic reactions, the position of the equilibrium makes it appear that the reactions have gone to completion. In a handful of reactions, such as acetal formation, esterification, and, most importantly, acid/base chemistry, chemical equilibrium are vitally important. This is summary was written from the point of view of a synthetic chemist. It covers the basics. It doesn’t get bogged down with activities and is, embarrassingly, a little woolly with its use of standard conditions°. My very poor justification for this is that I’m trying to get the basic concepts across … I need some of this for a discussion of acids/bases and then reactions but I don’t need a huge amount of detail!
There will be a subsequent post covering Le Chatelier controlling the reaction outcome.
Stereochemical descriptors 3: Assigning a descriptor to a stereogenic plane
Having covered how to assign a stereochemical descriptor of both stereogenic centres and axes, it seemed to make sense to finish the organic stereochemical nomenclature with the descriptors for stereogenic planes (obviously, there is still plenty of nomenclature out there for people who like that kind of thing … metal complexes and some of the wonderful supramolecular constructs).
Annoyingly, there are multiple naming conventions for stereogenic planes with cyclophanes (and similar compounds) being treated differently from metallocenes. Worse, both of these categories can be further subdivided depending on use of stereocentres vs helices or just different naming systems being used simultaneously.
But here goes, hopefully it will be useful …
Stereochemical descriptors 2: Assignment of configuration for helical and axial chirality
Most chemists are reasonably happy assigning the stereochemical descriptors R or S to stereocentres, tetrahedral atoms with four different substituents. That confidence starts to drop off when we turn our attention to molecules with other ‘forms’ of chirality, be they planar, axial or helical chirality. This summary introduces how to determine the configuration of helices (using the descriptors P or M) and compounds with a stereogenic axis (using either the R/S terminology or treating them as a helix and using P/M).
An Introduction to the Conformation of Cyclohexane
Cyclohexane is the only strain-free cycloalkane. Understanding the chair conformation of cyclohexane, with its two steric environments depending on whether groups are axial and equatorial is an important skill. It can help you understand the shape adopted by biomolecules such as the carbohydrates. At more advanced levels, it forms the basis for simple transition models of many reactions and is used to justify the stereochemical outcome of reactions. This summary covers the basics, how to draw the chair conformation and how to place subsituents around the ring.
Conformations of simple acyclic alkanes
Molecules are not static. Single bonds can rotate causing the shape of a molecule to change. This is known as changing the conformation of a molecule. Some conformations are more favourable than others, these are sometimes called conformers. Other conformations are disfavoured. They are the barrier to rotation or a transition state between conformers. This summary looks at the conformations of simple alkanes.
Isomer Flowchart
The classic, what kind of isomer do I have here flowchart. There are a number of these online but it is useful. Surprisingly, hard to fit onto one page (nicely).
Stereochemical Descriptors: Naming Molecules
The name of a molecule must describe its structure including any elements of stereochemistry. This post introduces stereochemical descriptors for alkenes and simple stereocentres. Although I no longer teach this at undergraduate level, it should be useful for those that do. I’ve also thrown in some tips on manipulating drawings containing stereochemistry.
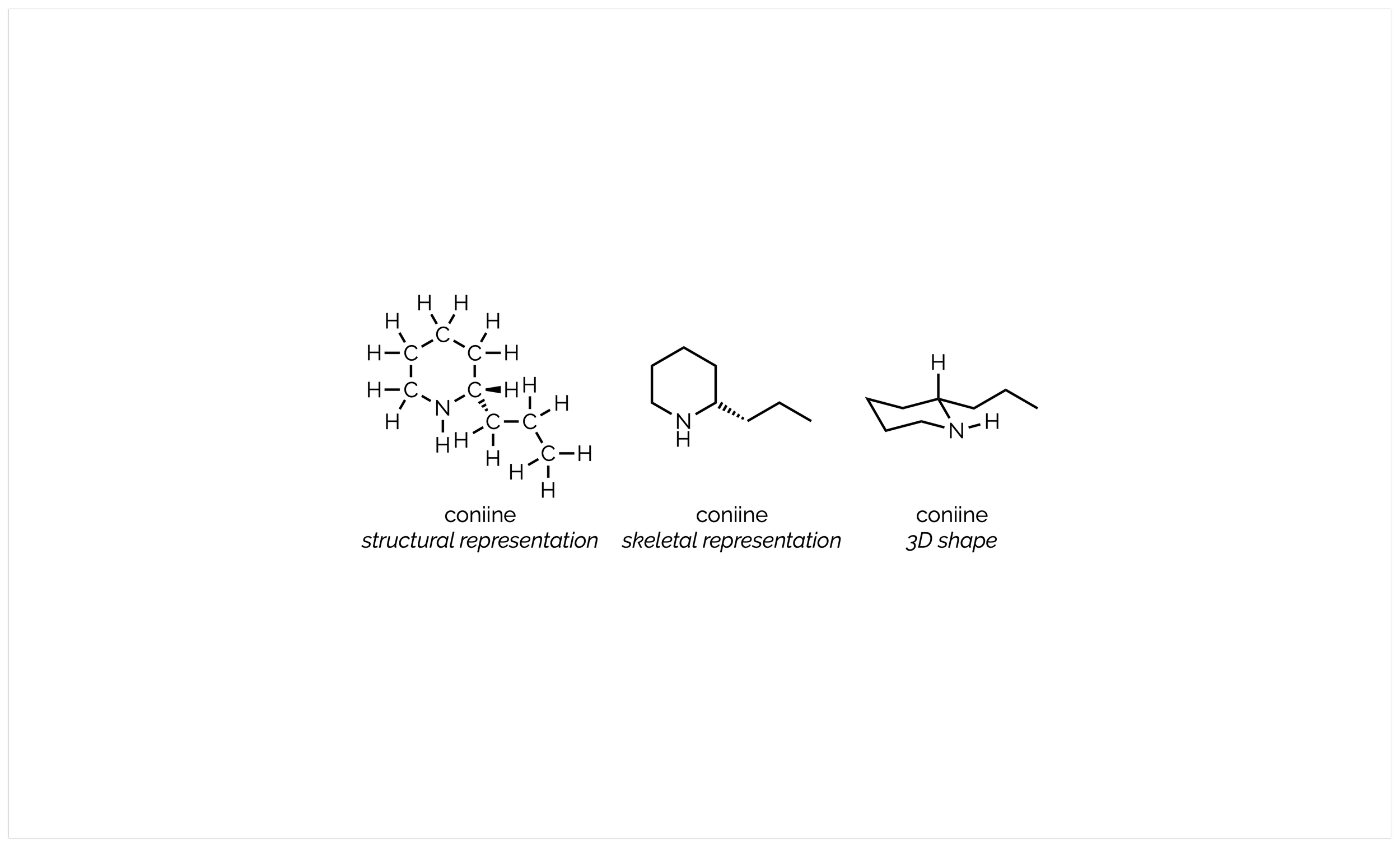
An Introduction to Stereochemistry
The shape of molecules is vital to understanding their chemistry and properties. The simple geometry of each atom is only half the picture, and you must also consider stereoisomers. These are compounds that have all the same atoms and bonds yet are different due to their shape. Students often find this a daunting topic. This introduction just lays out the basics. Sometime in the future, I’ll go into more detail (as I do teach a postgraduate course on this topic!)
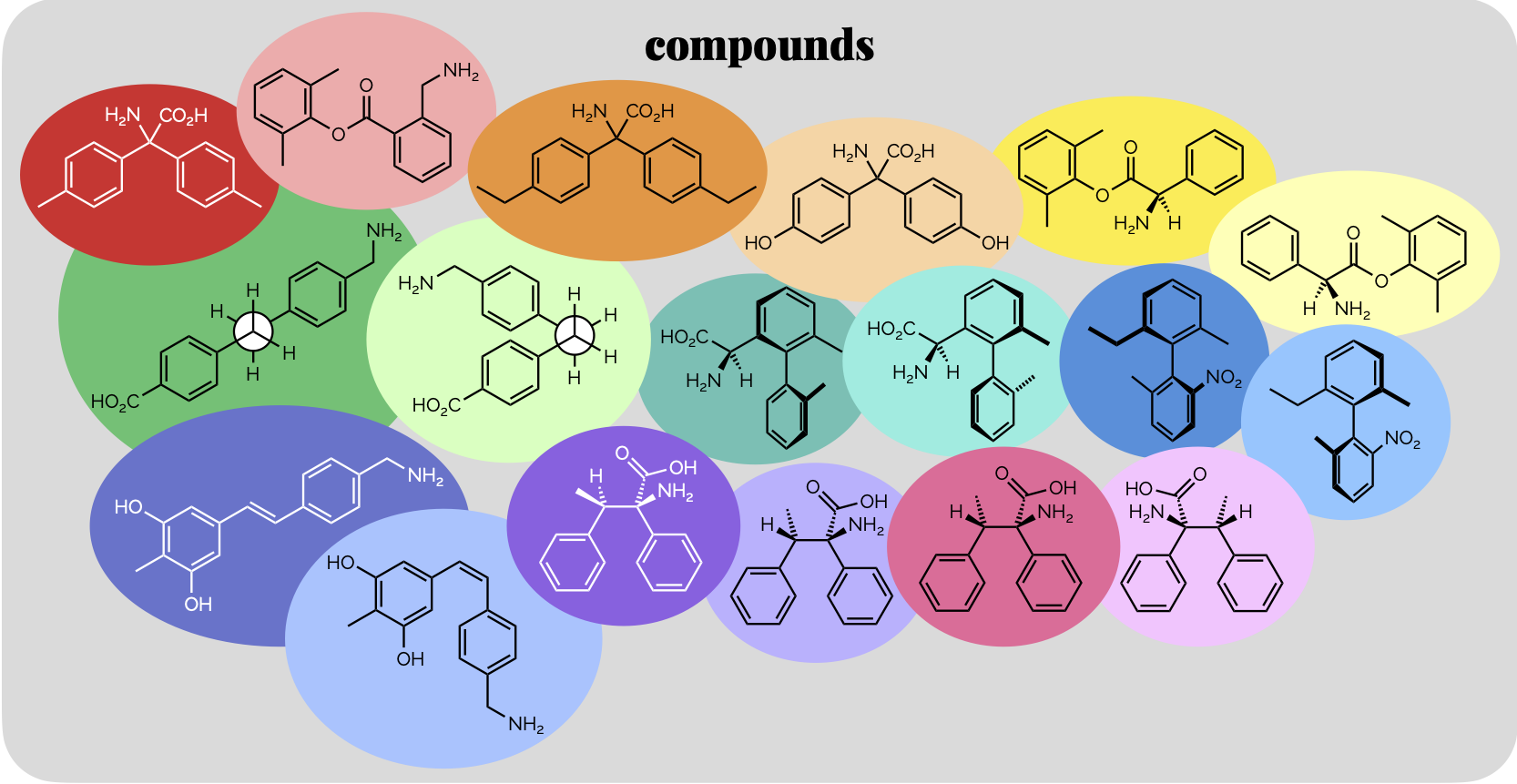
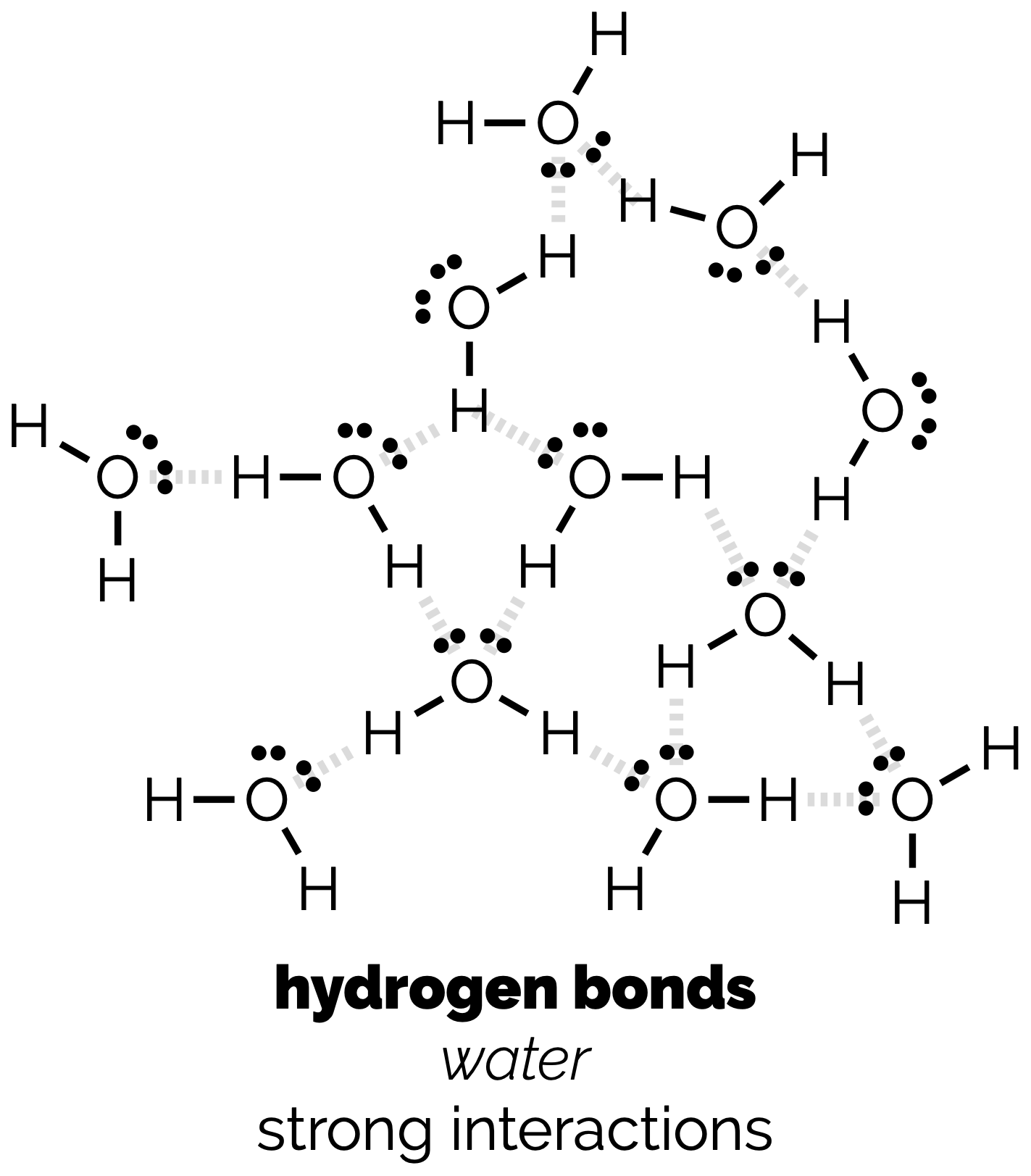
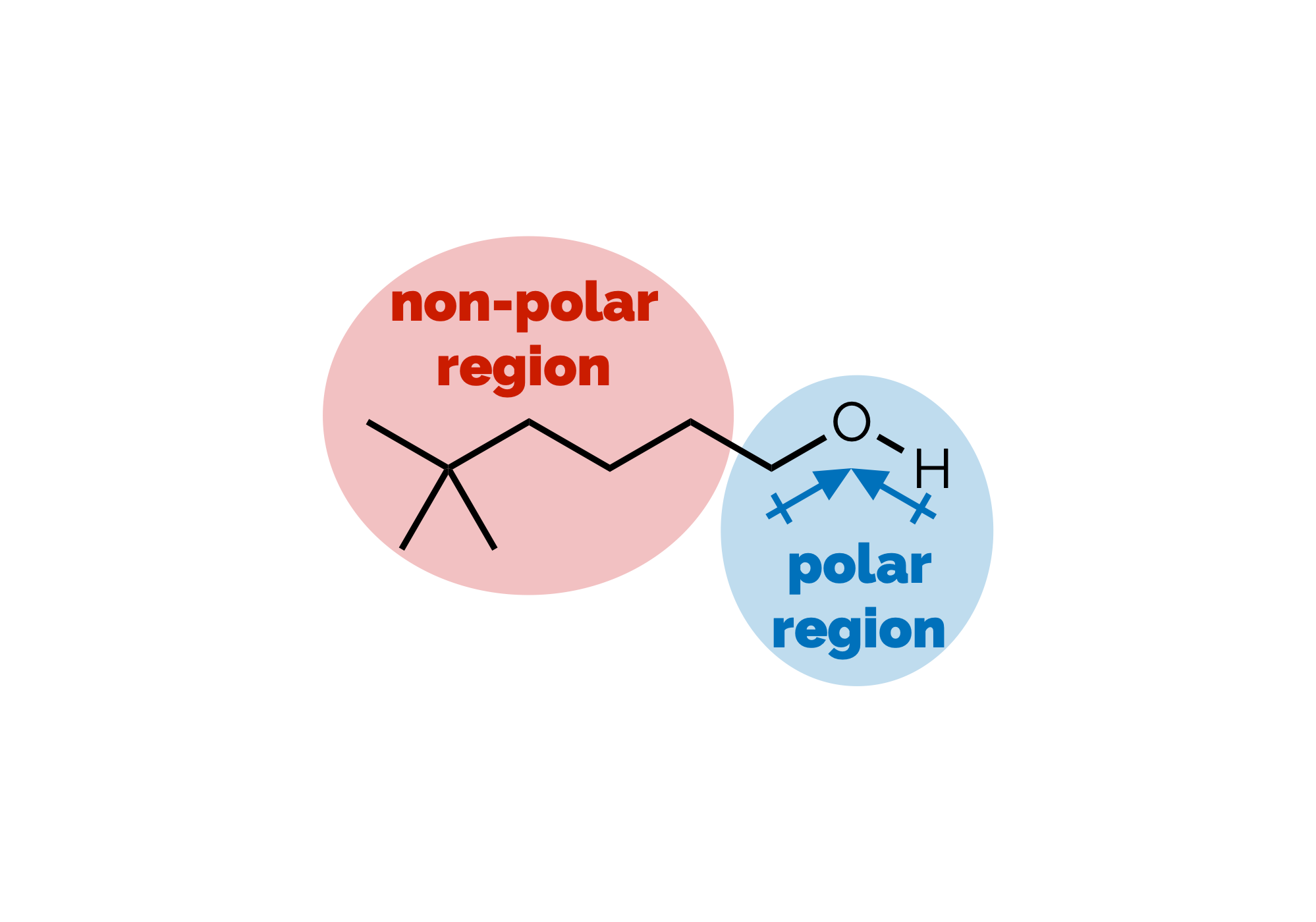
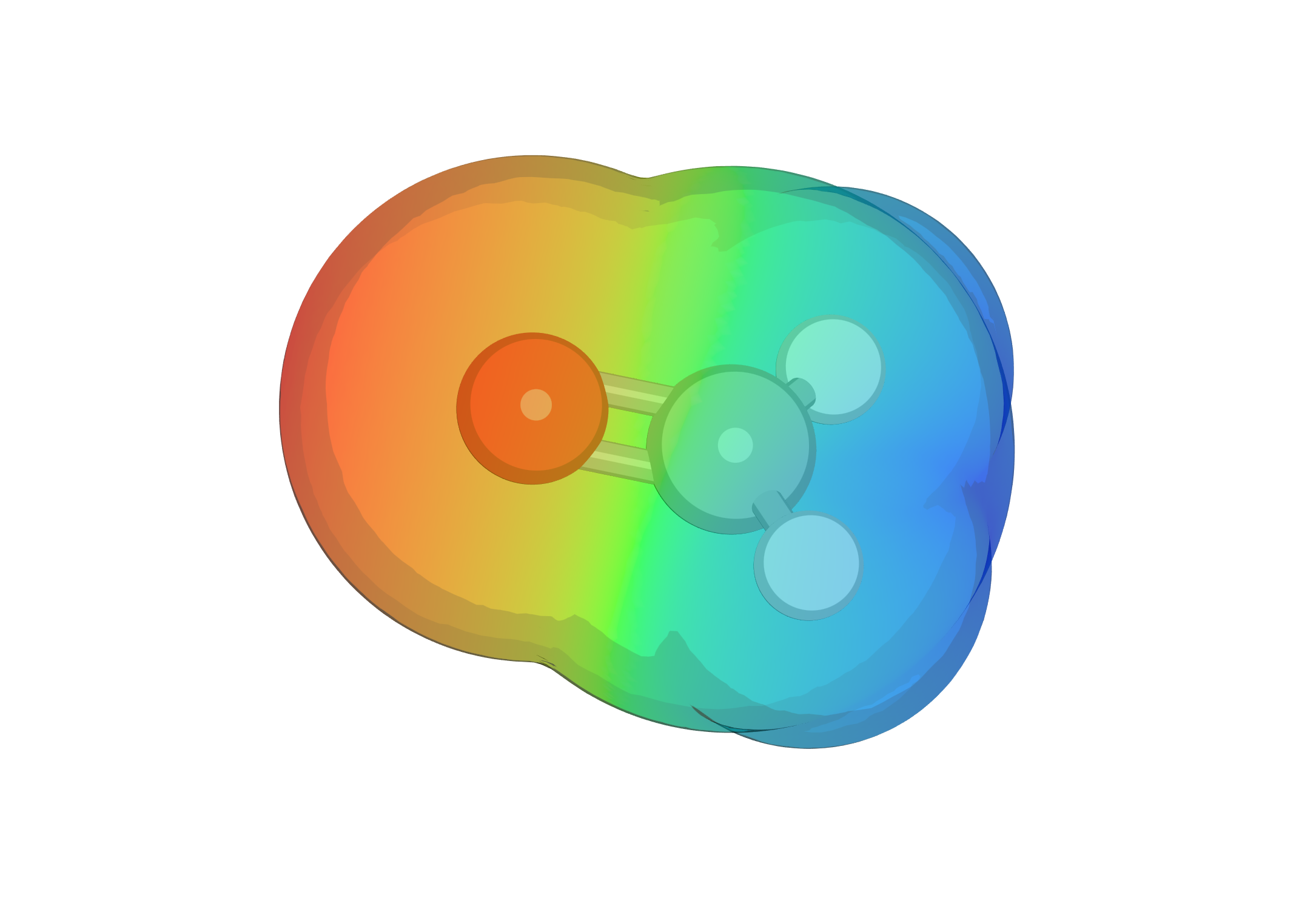
Polarity Part 2: Intermolecular Interactions & Physical Properties
A continuation of the last post in which we discussed the various non-covalent interactions between molecules. This post looks at some of the simpler consequences of these interactions.
Polarity & Non-Covalent Interactions Part 1
The uneven distribution of electrons within a molecule is vital to understanding reactivity and physical properties. These subjects, polarity and then the interactions between molecules, are frequently lumped together under the term ‘intermolecular forces’. As some of the most important examples of such forces occur within single (large) molecules we’re going to avoid that term (and don’t get our physical chemistry colleagues started on the meaning of ‘forces’). This first blog post will look at polar bonds and non-covalent interactions. Then, in a second, we’ll discuss how the interactions can be used to predict certain physical properties.
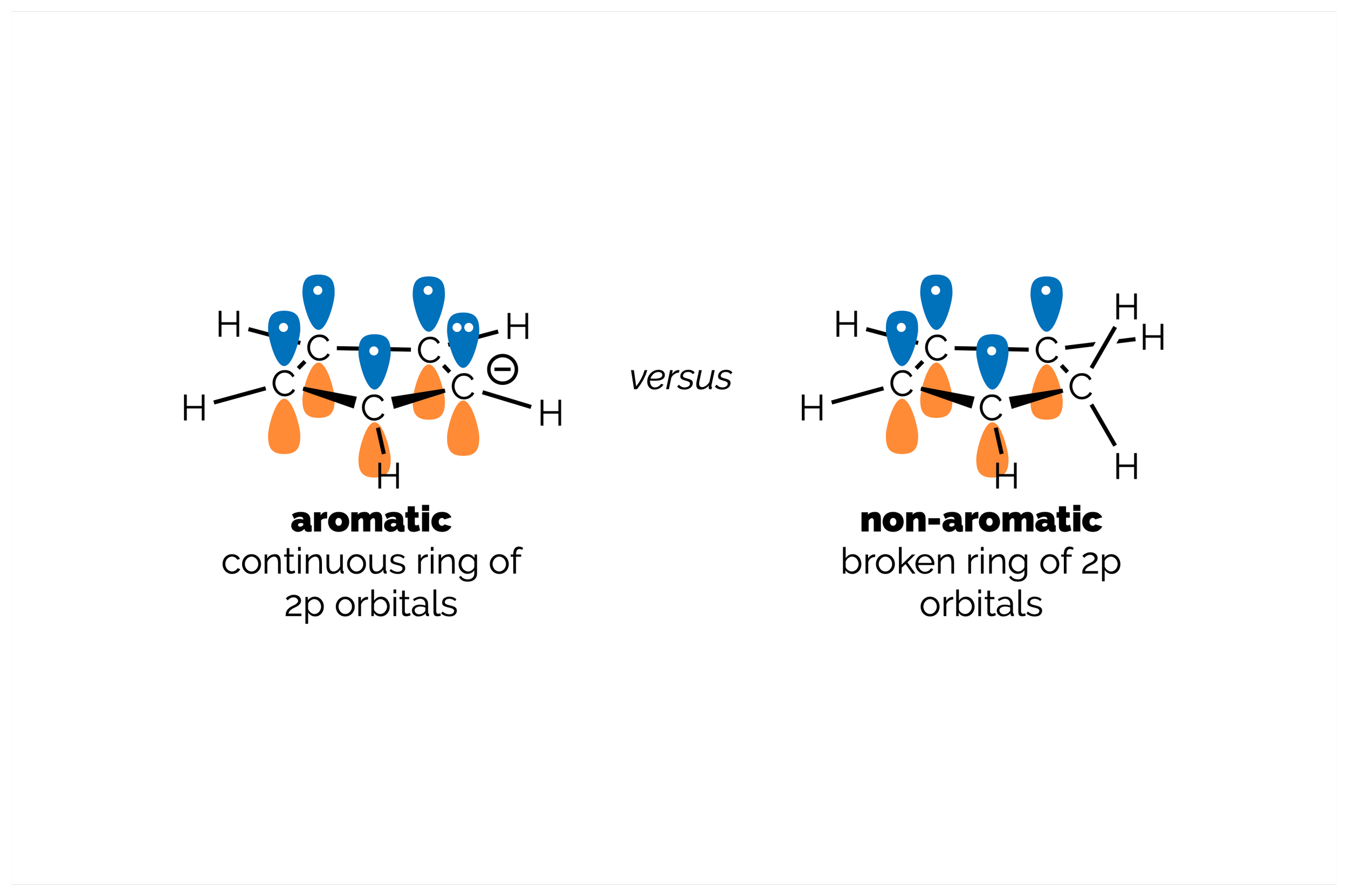
Aromatic Molecules
What makes a molecule aromatic? This summary shows you how to identify an aromatic ring, focusing on counting π electrons (as this is can cause some confusion).
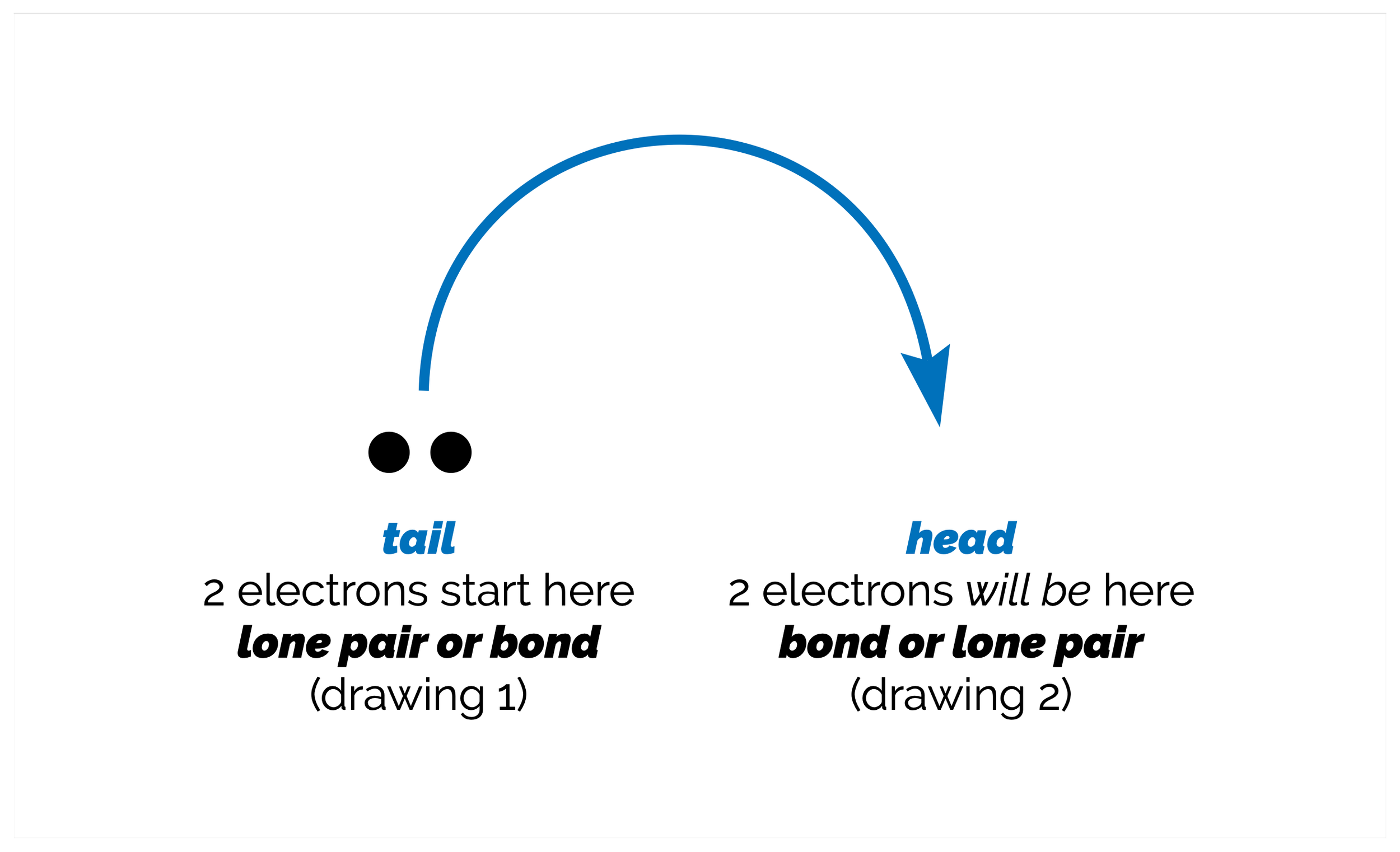
Resonance Structures & the Curly Arrow
Our second look at resonance structures and an introduction to the curly arrow notation. Curly arrows are a powerful tool for organic chemists, helping us predict reactions. The downside is that students hate them as they take a while to understand, hence this summary (and at least one other in the future).
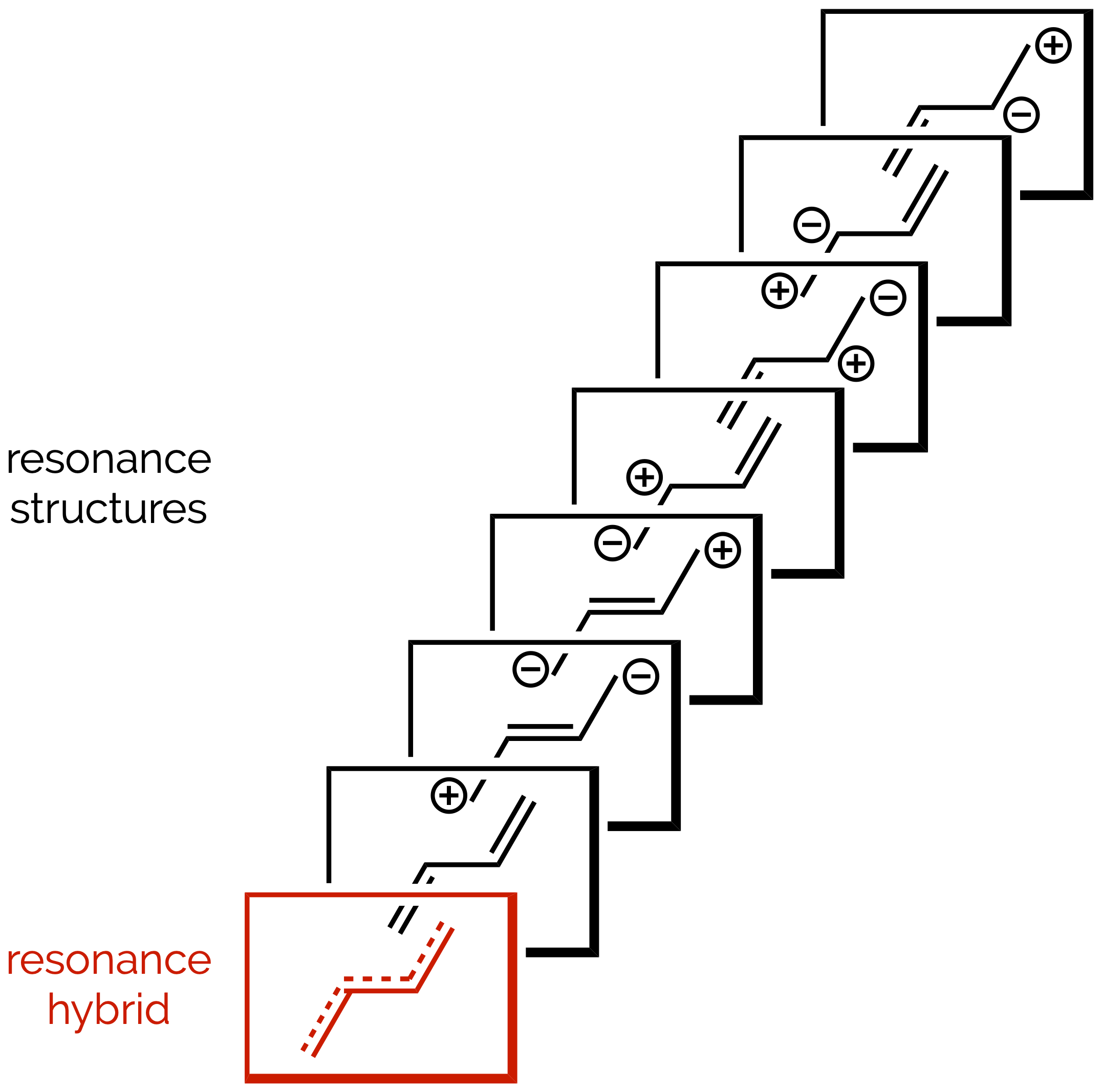
Delocalization & Resonance
Undergraduates often struggle with the delocalization of electrons and the subject of resonance. This is understandable, it is a fudge-factor required so that chemists’ simple line diagrams better represent real molecules. Here we try to demystify this topic, but undoubtedly cause more confusion!
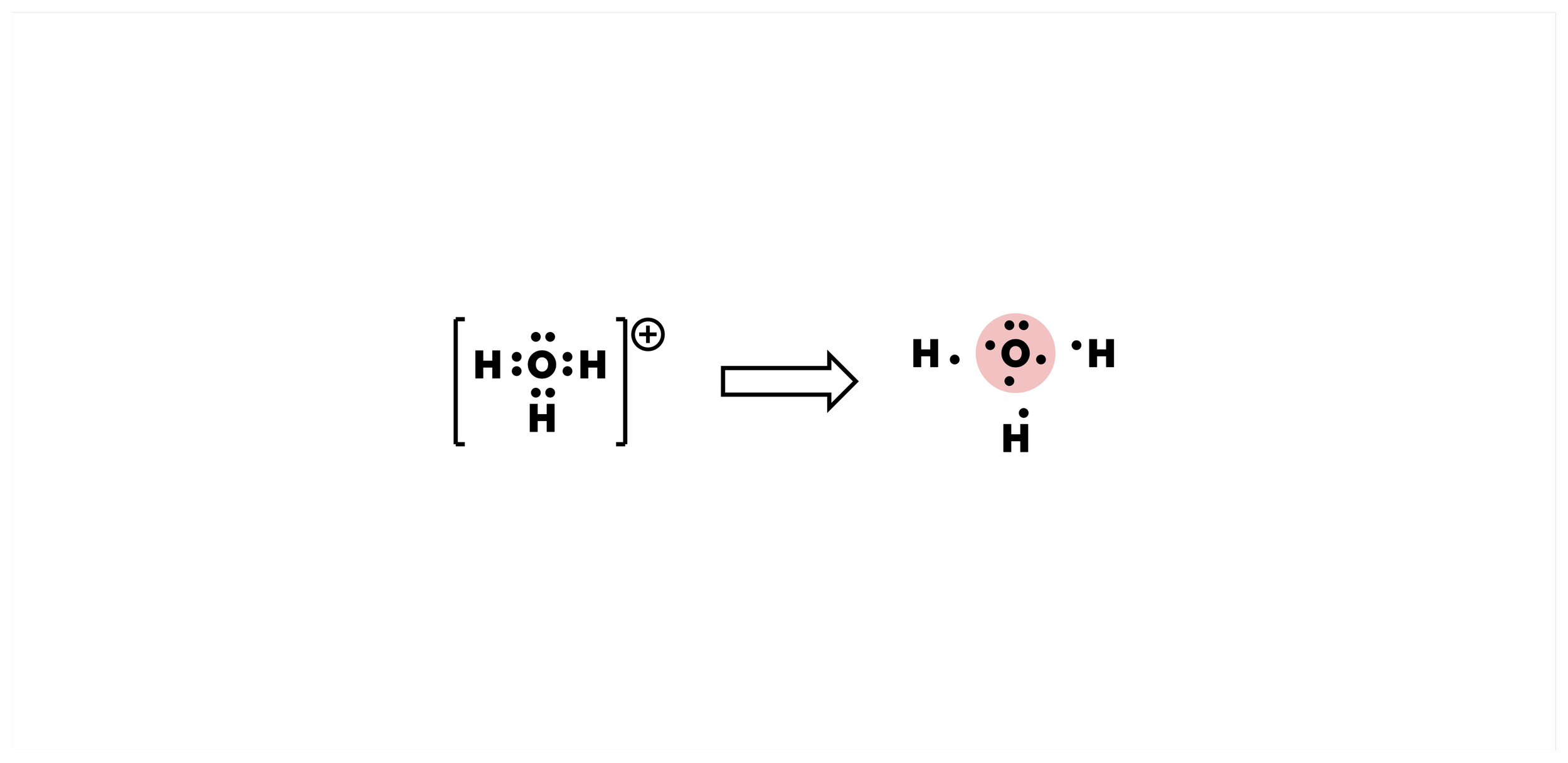
Formal Charges
Formal charges act as a signpost to indicate which atoms are sharing (or not) their electrons in an unusual manner in a Lewis structure. They give more insight into electron distribution and hence reactivity and physical properties.
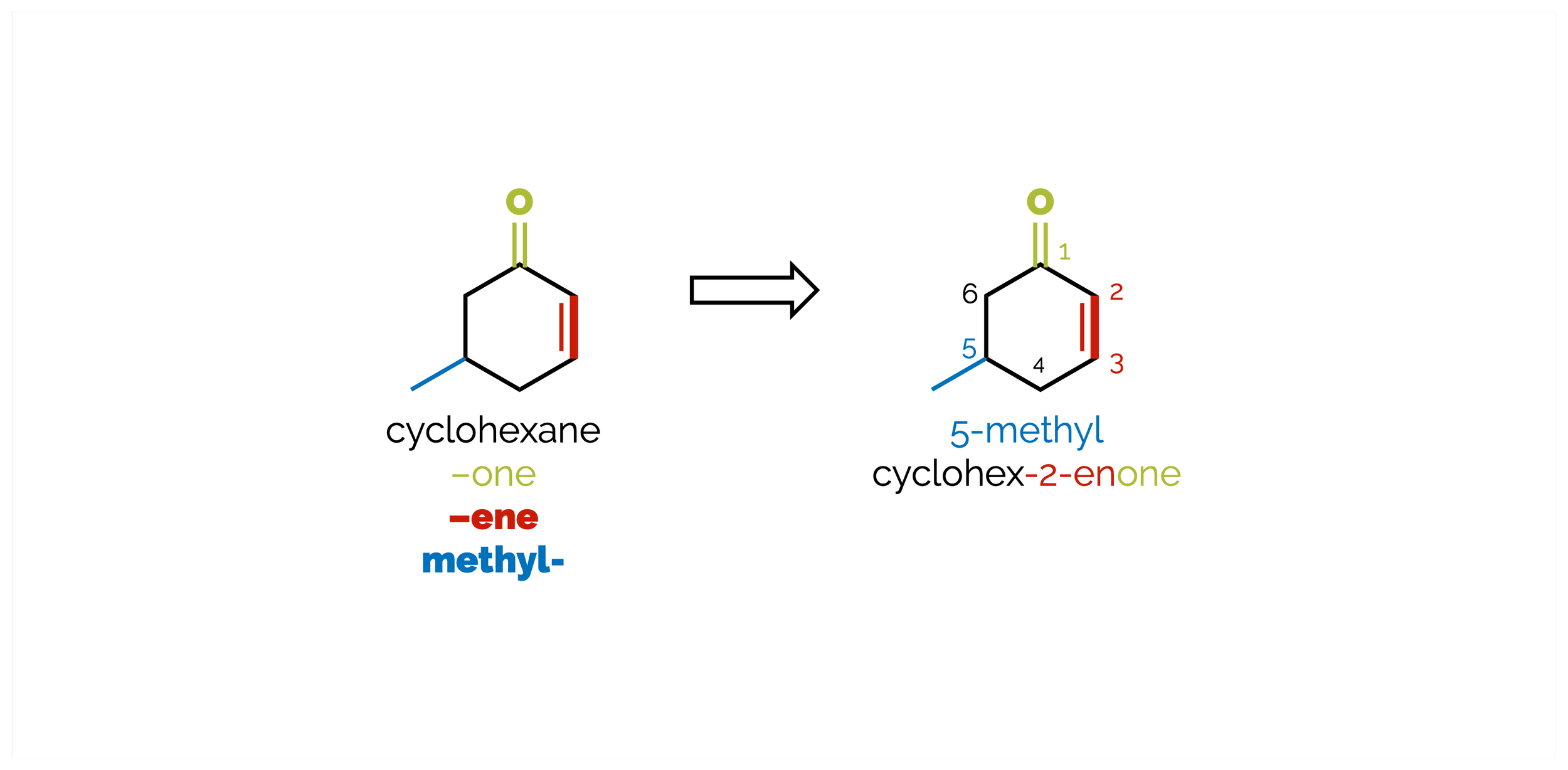
Naming Molecules
A brief introduction to organic nomenclature. A bit longer than I hoped as I added some examples (pluses and minuses I guess).
Hybrid Atomic Orbitals & Valence Bond Theory
An overview of hybrid atomic orbitals and how they are applied to valence bond theory.
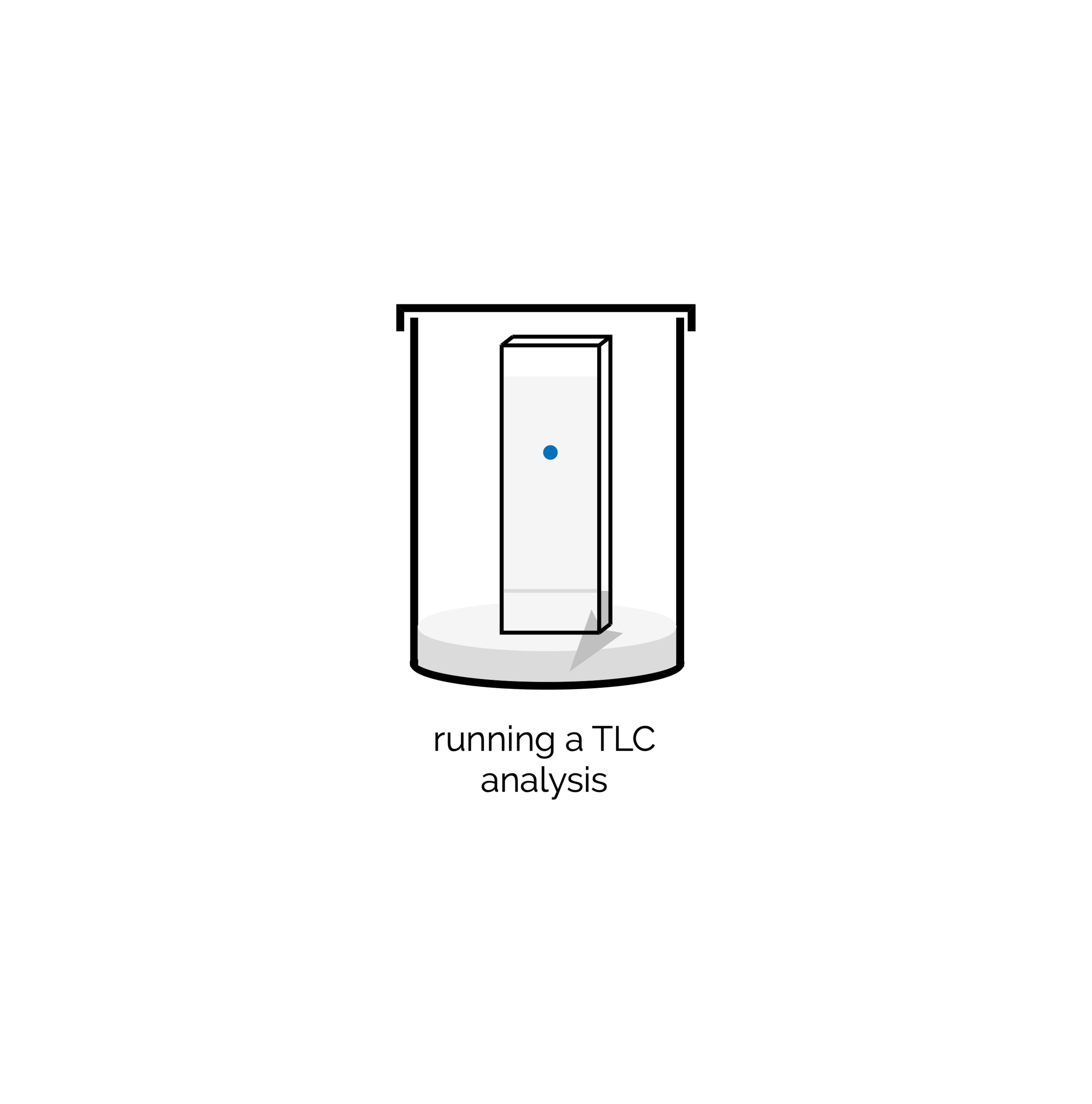
Thin Layer Chromatography (TLC)
A quick introduction to thin layer chromatography - hopefully useful for first year labs.